To the Editor
Adamantinoma is a rare tumor that affects long bones, and it is estimated to account for 0.1 to 0.5% of all primary bone tumors [Citation1]. It is named for its histological similarity to the tumor of the mandible (ameloblastoma). Although its origin remains controversial, recent opinion seems to suggest that adamantinoma is a tumor of epithelial origin, based on ultra structural and immunohistochemical studies [Citation2–4]. Only approximately 200 cases of adamantinoma have been reported since the tumor was first described by Fischer in 1913 [Citation5]. The tumor occurs almost exclusively in long bones, with tumors in the tibia accounting for more than 80% of cases.
It is a low-grade neoplasm that presents an indolent course, is locally aggressive and rarely metastasizes. Appropriate surgical treatment may lead to 10-year survival rate of 89.2% [Citation6]. The secondary sites most frequently affected are the lungs [Citation7], lymph nodes and bones. Reported risk factors associated with metastatic disease are: male gender, pain, symptoms of less than 5 years’ duration and histologic feature of squamous differentiation [Citation8]. We report a rare case of adamantinoma metastatic to the lung with documented overexpression of c-kit, PDGFR-beta and VEGF-R, and responding to tyrosine kinase inhibitor.
Case report
A 56-year-old man presenting with left-side chest pain, hypercalcemia and pulmonary tumors was referred to the University of Minnesota Masonic Cancer Clinic. The patient had previous diagnosis of adamantinoma of the right tibia in 2000. Prior to this diagnosis, the patient was involved in a motorcycle accident in 1990 that resulted in a complex fracture of his right foot. His clinical course was uneventful until 1999, when he developed pain in the right lower extremity and was found to have a tumor growing in the lower part of the right tibia. With the discovery of the tumor, the patient had below-knee amputation. Past medical history was also significant for psoriasis, vitamin B12 deficiency requiring administration of intramuscular B12 injections and a history of appendicitis. The patient quit smoking in 1974, but smoked for several years prior to quitting. Family medical history was negative for cancer or other significant disease.
The patient recovered from surgery and followed a relatively normal course over the next eight years. In 2008, he developed significant weight loss of several pounds accompanied by minimal appetite. The patient also reported experiencing left-side chest pain during the previous few months, shortness of breath on exertion and coughing productive of mostly clear phlegm, which was occasionally tinged with blood. The diagnostic work-up revealed hypercalcemia, which was treated with zolendronic acid.
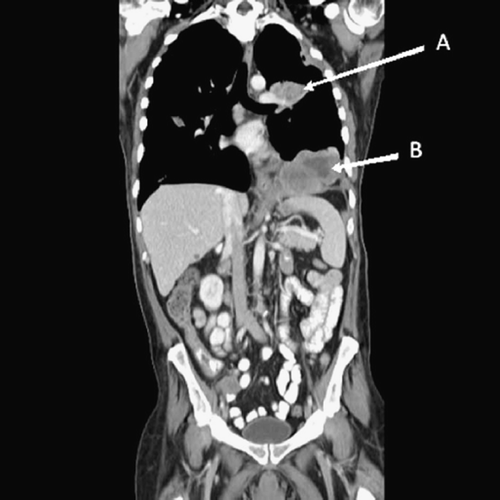
Chest computed tomography (CT) scan with intravenous contrast demonstrated the presence of multiple pulmonary nodules. A left pleural-based mass (6.9 × 7.1 cm) with mixed density and predominately low attenuation exerted a mass effect on the adjacent left upper lobe pulmonary artery, causing complete occlusion of this vessel, as well as a mass effect on the left upper lobe bronchus, causing atelectasis of the left upper lobe, volume loss in the left lung and dilatation of the distal bronchi and air bronchograms (). A left diaphragmatic mass (14 × 10 cm) with mixed density and predominately low attenuation produced a mass effect on the left hemidiaphragm pushing it inferi-orly, and a mass-effect on the adjacent heart with deviation towards the midline (). There were bilateral pleural effusions with associated atelectasis.
Figure 1. CT scan performed on 2/26/08 prior to sunitinib therapy. The pleural (A) and diaphragmatic (B) tumors measured 89 mm and 105.5 mm, respectively, in the vertical dimension.
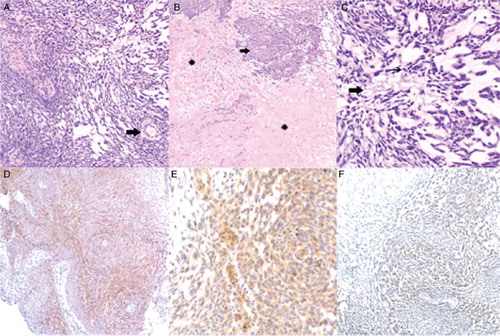
Biopsies of the pulmonary nodules were performed at the University of Iowa and were positive for histology similar to primary adamantinoma. The biopsy specimen of the primary tumor from distal right tibial lesion resected in 2000 was then analyzed at the University of Minnesota. Sections showed a spindle cell neoplasm comprised of interspersed cellular areas with a storiform architectural pattern and thick-walled blood vessels, paucicellular myxoid areas, and areas of fibrosis and hyalinization (, ). The tumor cells had scant cytoplasm and plump, vesicular, moderately atypical nuclei. There were approximately 10 mitotic figures per 10 high power fields in the most mitotically active areas (). The tumor showed focal positive immunostaining for CD117 (c-kit) and vascular endothelial growth factor receptor-2 (VEGFR-2), with some cancer cells staining weakly for platelet-derived growth factor receptor beta (PDGFR-beta) (). No mutation was detected in KIT exons 9,11,13,17 and 18. Based on overexpression of VEGF, PDFR-beta and c-kit, the patient was treated with sunitinib 50 mg daily for 4 weeks on and 2 weeks off for 4 cycles.
Figure 2. Histological analysis of the primary adamantinoma tumor. (A) Cellular and myxoid areas of the tumor. Scattered thick-walled blood vessels were noted (arrow). Hematoxylin and eosin; original magnification, ×200. (B) Hyalinized (asterisks) and cellular (arrow) areas of the tumor. Hematoxylin and eosin; original magnification, ×100. (C) Nuclear features of the tumor. Occasional mitotic figures (thin arrow) and focal necrosis (thick arrow) were noted. Hematoxylin and eosin; original magnification, ×400. (D) Focal positive immunostaining of the tumor for CD117 (c-kit). CD117 immunostain; original magnification, ×200. (E) Focal positive immunostaining of the tumor for platelet-derived growth factor receptor beta (PDGFR-beta). PDGFR-beta immunostain; original magnification, ×400. (F) Focal positive immunostaining of the tumor for vascular endothelial growth factor receptor-2 (VEGFR-2). VEGFR-2 immunostain; original magnification, ×200.
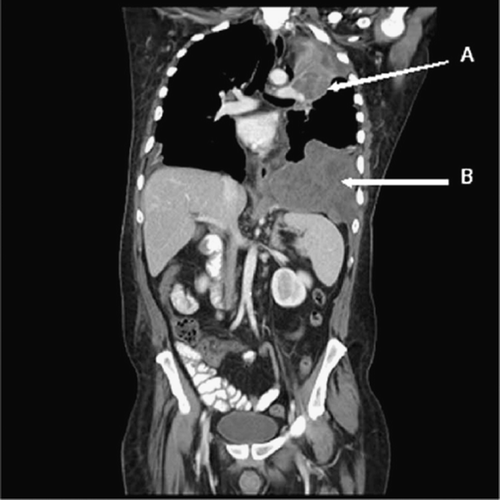
CT scan performed after one cycle of sunitinib therapy showed a decrease in the size of the left pleural-based mass to 6.3 × 6.0 cm (vs. 6.9 × 7.1 cm) and left diaphragmatic mass to 12 × 9 cm (vs. 14 × 10 cm). The patient had significant improvement in shortness of breath and cough. CT scan done after 4 months of therapy demonstrated sustained disease control (). At the last visit after 11 months of sunitinib therapy, disease remains stable and the patient has regained normal weight and is free of clinical symptoms.
Discussion
Sunitinib inhibits cellular signaling by targeting multiple RTKs. These include PDGF-R isoforms, VEGF-R and c-kit receptor (CD117) which play a role in both tumor angiogenesis and tumor cell proliferation. The simultaneous inhibition of these targets, therefore, leads to both reduced tumor vascularization and cancer cell death, and ultimately tumor shrinkage. Metastatic adamantinoma cases are extremely rare and usually treated surgically. Effective systemic therapy of unresectable metastatic adamantinoma has only been reported once before for a case of metastatic adamantinoma of tibia to the lung [Citation9]. Tumor regression was observed in response to a combination of etoposide plus cisplatin or carboplatin; however, treatment had to be discontinued due to poor tolerance. During the subsequent 4 months, the patient's lung tumors increased in size and were unresponsive to other forms of chemotherapy, including ifosfamide, car-boplatin, methotrexate, fluoropyrimidines and doxorubicin. In another case, radiation therapy to chemithorax was used to control hypercalcemia associated with this disease [Citation10].
Conclusion
We conclude that adamantinoma – a rare, slow-growing neoplasm of bone that occasionally metastasizes to the lungs, as in the case presented here – may respond to treatment with the tyrosine kinase inhibitor, sunitinib. We posit that positive immune staining for c-kit, PDGFR-beta and VEGF-R may help in the selection of adamantinoma patients for this therapy.
Acknowledgements
The authors are grateful to Ignacio I. Wistuba from the University of Texas MD Anderson Cancer Center, Houston, TX, for performing the immunostain for VEGFR-2 on the biopsy tissue from this case.
References
- Gebhardt MC, Lord FC, Rosenberg AE, Mankin HJ. The treatment of adamantinoma of the tibia by wide resection and allograft bone transplantation. J Bone Joint Surg Am 1987;69:1177–88.
- Van Rijn R, Bras J, Schaap G, van den Berg H, Maas M. Adamantinoma in childhood: Report of six cases and review of the literature. Pediatr Radiol 2006;36:1068–74.
- Desai SS, Jambhekar N, Agarwal M, Puri A, Merchant N. Adamantinoma of tibia: A study of 12 cases. J Surg Oncol 2006;93:429–33.
- Jundt G, Remberger K, Roessner A, Schulz A, Bohndorf K. Adamantinoma of long bones. A histopathological and immunohistochemical study of 23 cases. Pathol Res Pract 1995;191:112–20.
- Fischer B. Uber ein primares Adamantinom der Tibia. 12. Frankfurt: Zeitschr. f. Path. 1913;422–41.
- Qureshi AA, Shott S, Mallin BA, Gitelis S. Current trends in the management of adamantinoma of long bones. An international study. J Bone Joint Surg Am 2000;82:1122–31.
- Szendroői M, Antal I, Arató G. Adamantinoma of long bones: A long-term follow-up study of 11 cases. Pathol Oncol Res 2008;Dec 2 Epub.
- Keeney GL, Unni KK, Beabout JW, Pritchard DJ. Adamantinoma of long bones. A clinicopathologic study of 85 cases. Cancer 1989;64:730–7.
- Lokich J. Metastatic adamantinoma of bone to lung. A case report of the natural history and the use of chemotherapy and radiation therapy. Am J Clin Oncol 1994;17:157–9.
- Lyons JA, Budd GT, Crownover RL. Hypercalcemia caused by metastatic adamantinoma: Response to radiotherapy. Sarcoma 1999;3:33–5.