Neuroblastoma is the most common extracranial cancer in children. High risk cases are often treatment resistant and result in significant mortality. Spontaneous regression is only seen in the 4S type characterized by young age (under one year), skin involvement and localized, operable disease. We report a six-year-old boy with high risk non-4S Stage 4 disease metastatic to lymph nodes and bone marrow. He had previously been treated with three different chemotherapy regimens (vincristine + cisplatin/carboplatin + etoposide + cyclophosphamide, doxorubicin + etoposide + iphosphamide, high-dose cyclophosphamide) including a high-dose intensive regimen with an autologous stem cell transplant. These treatments, however, had little effect on tumor load and disease continued to progress.
The patient was treated with an ultrasound guided injection of 10e11 viral particles of Ad5/3-Cox2L-D24, an advanced generation oncolytic adenovirus, one into the primary tumor near the left kidney [Citation1], into adjacent lymph nodes and intravenously. Written informed consent was obtained from the patient's parents before treatment. Compassionate use of oncolytic adenovirus is in accordance with the Declaration of Helsinki and was approved by the Finnish Gene Technology Board, Department of Medicolegal Affairs and Ministry of Basic Services. Oncolytic viruses are replication competent in tumor but not normal cells and cancer cells are killed by replication per se [Citation2]. Ad5/3-Cox2L-D24 features a chimeric capsid for enhanced gene delivery to cancer cells. Tumor specific replication is achieved by two additional modifications, the cyclo-oxygenase 2 (Cox2) promoter driving E1A gene and a 24 bp deletion in Rb binding site of E1A [Citation1].
Treatment resulted in grade 1 fever (max 37.1C), diarrhea, stomach pains and grade 2 liver enzyme elevations for two weeks. One month after treatment, a bone marrow aspirate was free of disease for the first time since diagnosis, and the primary tumor had regressed by 71% (). S-NSE, dU-HVA, dU-Moma, and dU-Noradr values were low after treatment, while they had been elevated previously.
Figure 1. A chemotherapy resistant case of neuroblastoma was treated with oncolytic adenovirus Ad5/3-Cox2L-D24. A) Computer tomography of primary tumor before oncolytic virus treatment. B) Magnetic resonance imaging one month after treatment shows moderate decrease of the primary tumor and complete regression of the lymph nodes. C) Before treatment, bone marrow was almost completely infiltrated by neuroblastoma cells (CD56 staining, brown, 10x), (monoclonal mouse anti-SCLC (CD56, N-CAM), Clone 123C3, Invitrogen, CA, USA). D) Few neuroblastoma cells in bone marrow three months after oncolytic virus treatment (10x). E) Oncolytic virus treatment increased the number of CD3+/CD8+ cytotoxic T-cells in blood. F) Anti-adenoviral neutralizing antibodies were induced. G) Virus was detected in blood for three weeks after treatment. H) Immunohistochemistry indicated intermediate Cox2 expression (brown, 20x) in tumor cells (monoclonal mouse anti-human Cox-2, Clone CX229, Cayman Chemical, MI, USA).
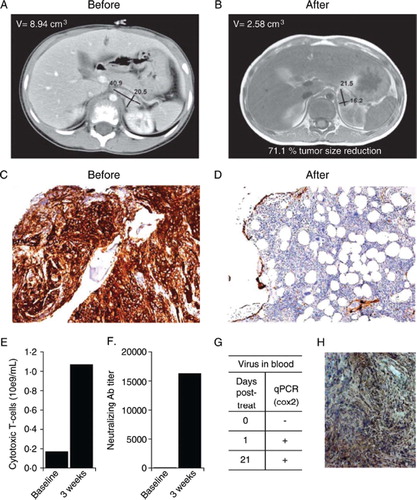
Oncolytic replication may be able to induce anti-tumor immunity and break immunological tolerance [Citation3]. Therefore, it was of interest that CD3+/CD8 + cytotoxic lymphocytes increased after treatment with Ad5/3-Cox2L-D24 (). These cells might have been induced toward tumor or viral epitopes or both [Citation4]. Despite the rapid induction of neutralizing antibodies (), virus was nevertheless detected in blood for three weeks after treatment, suggesting effective replication and humoral dissemination (). Intermediate Cox2 expression was seen in the tumor, which may have facilitated replication of Ad5/3-Cox2L-D24 ().
Imaging was repeated three months after the treatment with identical results, while tumor markers remained low. At three months, a bone marrow biopsy indicated a minimal number of tumor cells in a mostly healthy marrow (). The patient remains alive and in good health 14 months after treatment. We conclude that Ad5/3-Cox2L-D24 may be promising for treatment of chemotherapy resistant neuroblastoma, and should be tested in the context of a clinical trial.
References
- Bauerschmitz GJ, Guse K, Kanerva A, Menzel A, Herrmann I, Desmond RA, . Triple-targeted oncolytic adenoviruses featuring the cox2 promoter, E1A transcomplementation, and serotype chimerism for enhanced selectivity for ovarian cancer cells. Mol Ther 2006;14:164–74.
- Liu TC, Galanis E, Kirn D. Clinical trial results with oncolytic virotherapy: A century of promise, a decade of progress. Nat Clin Pract Oncol 2007;4:101–17.
- Alemany R. A smart move against cancer for vaccinia virus. Lancet Oncol 2008;9:507–8.
- Prestwich R, Harrington K, Vile R, Melcher A. Immunotherapeutic potential of oncolytic virotherapy. Lancet Oncol 2008;9:610–2.
