Abstract
Background. Signet-ring cell carcinoma (SRCC) is an uncommon tumor entity in rectal cancer, often considered to be resistant to non-surgical therapy. In locally advanced primary or recurrent rectal cancer, diagnostic information from magnetic resonance imaging (MRI) is considered superior in planning the optimal treatment strategy, which usually includes preoperative radiotherapy. The recognition of MRI features that correlate with the radiation response might ultimately be used to select patients for tailored treatment and, in addition, avoid potentially toxic therapy in non-responding patients. Material and methods. In a cohort of 120 rectal cancer patients who had received preoperative radiotherapy (50 Gy in 2 Gy fractions), six patients were noted to have SRCC tumor differentiation. Initial diagnostic MRI examination included assessment of local T- and N-stage and tumor morphology. Histological tumor response was subsequently assessed in the resected specimens, and postoperative follow-up data was compiled until disease recurrence. Results. Following the preoperative radiotherapy, two distinctly different histological responses – complete response (ypT0N0) or no response – were observed. Extensive mesorectal lymph node metastasis (N2 disease) at the pretreatment MRI examination was unambiguously associated with lack of response and rapid development of disseminated disease. Importantly, patients with complete response have been observed for 23–52 months postoperatively without evidence of recurrent disease. Discussion. Our review may suggest that patients with locally advanced growth of rectal SRCC, despite poorer outcome when compared to patients with conventional-type rectal adenocarcinoma, when presenting limited lymph node disease should be offered preoperative radiotherapy in a tentatively curative setting.
Surgical resection remains the principal treatment modality in achieving complete tumor clearance in primary rectal cancer; however, several randomized trials have clearly highlighted the central role of neoadjuvant radiotherapy in optimizing local tumor control. Considerable improvements in treatment outcome have also been reported for more locally advanced rectal cancers, also where tumor extension beyond the mesorectal compartment with infiltration of adjacent pelvic organs is evident [Citation1].
Locally recurrent rectal cancer following primary surgery is a particularly difficult therapeutic challenge, and radical treatment options with curative intent have not been widely accepted [Citation1]. Nevertheless, our institution has recently reported on a five-year survival rate of 44% following preoperative radiotherapy and radical resection of recurrent tumor where clear resection margins are achieved [Citation2].
Locally advanced primary and recurrent rectal cancer in our institution is managed by a dedicated, multidisciplinary team, with formal assessment of individual patient eligibility for preoperative radiotherapy followed by radical surgery. Selection criteria for this approach include patients with poor-risk primary cancer with tumor extension and/or pathological lymph nodes within 3 mm or beyond the mesorectal fascia or patients with intrapelvic, local tumor recurrence eligible for definitive surgery, as determined by magnetic resonance imaging (MRI), with no additional evidence of chest or abdominal systemic disease on radiological examination.
MRI is the ‘gold-standard’ for staging of malignant disease extension within the pelvic cavity [Citation3]. In addition to providing precise information on T- and N-stage, more subtle features of tumor morphology (including signal patterns typical for particular tumor entities) and the presence of extramural vascular tumor invasion (tumor signal within or along extramural vessel contours) can also be assessed. Specific attention is placed on the recognition of extramesorectal lymph node metastasis (lateral spread of the tumor to pelvic sidewall nodes), including nodes along middle rectal vessels.
The primary treatment outcome is given by the degree of histological tumor regression and review of surgical margins; however, the relationship of this observation to future decreased risk of local or systemic disease recurrence has been an issue of some controversy [Citation1]. We examined whether any given features of pretreatment MRI might correlate with histological tumor response and the potential of such characteristics to select patients for neoadjuvant treatment for locally advanced tumors.
Signet-ring cell carcinoma (SRCC) is an uncommon histologic variant of rectal carcinomas, accounting for ∼1% of all colorectal malignancy in standard pathology series [Citation4–6], that is frequently diagnosed at an advanced disease stage and is associated with poorer patient outcomes when compared to other adenocarcinomas, including the mucinous subtype [Citation6,Citation7]. Although large prospective studies on preoperative radiotherapy for rectal cancer have included patients with SRCC, to our knowledge, data on therapy outcome for this patient subgroup has not been reported.
In this retrospective study, individual disease courses and outcomes of six patients with locally advanced rectal SRCC, either primary or recurrent, treated with preoperative radiotherapy, were reviewed. In particular, initial diagnostic MRI scans were reexamined to assess whether it was feasible to recognize rectal SRCC patients for the neoadjuvant radiation treatment strategy and the increased potential for long-term survival.
Material and methods
Study population
The Norwegian Radium Hospital (Oslo University Hospital) is a referral center for multidisciplinary management of locally advanced primary and recurrent rectal cancer. Within the study review period (April 2003–January 2006), 120 patients with histologically confirmed carcinoma of the rectum were deemed by the institutional multidisciplinary team to be candidates to receive preoperative radiotherapy before radical surgery. Within this patient cohort, six patients presented a tumor with SRCC signal pattern on the diagnostic, pelvic MRI examinations (see definition of diagnostic criteria below), which was in full accordance with the confirmed tumor entity (i.e., the histopathological characterization of more than 50% of the tumor cells containing intracytoplasmic mucin that displaces the nucleus to the cell's periphery within a poorly differentiated adenocarcinoma [Citation8]; ). For each patient, symptoms and any comorbidities were recorded. Since the study population received treatment and postoperative follow-up in accordance with standard protocols, this study was considered to represent a retrospective audit of six separate cases that did not require formal hospital ethics committee approval.
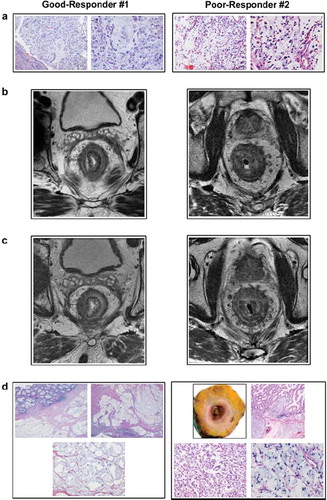
Figure 1. Tumor histology and MRI specimens – exemplified by Good-Responder #1 and Poor-Responder #2. (a) Diagnostic tumor biopsies, each in two magnifications, from rectum displaying SRCC differentiation. (b) Diagnostic MRI scans, axial and T2-weighted images, illustrating rectal tumors with SRCC signal pattern. (c) Preoperative MRI scans, axial and T2-weighted images, illustrating rectal tumors with SRCC signal pattern. (d) Surgical specimens, in several magnifications, depicting treatment responses.
MR examination and image interpretation
Pelvic MR examinations using validated protocols were completed on all patients [Citation9,Citation10], using a 1.5-T scanner, phased-array coil, and two-dimensional fast spin-echo T2-sequences before the commencement of treatment and within 4 weeks after completion of preoperative radiotherapy. High-spatial resolution, T2-weighted images were acquired in a plane perpendicular to the mesorectum at the site of tumor and any suspicious lymph nodes. In low rectal cancers, coronal imaging was also performed using similar scan parameters.
SRCC is prone to spread through the rectal wall without necessarily destroying involved anatomical structures. In this study, morphology of rectal SRCC was defined by MRI as concentric wall thickening with inhomogeneous T2 signal intensity, depicting the target sign of concentric tumor rings within the rectal wall, separating its anatomical layers ( and ). In common with other forms of rectal cancer, the extramural growth is typically within or along vessels traversing the rectal wall into the superficial vessel plexus of the mesorectum, where the superior and the medial rectal vessels are communicating.
As part of this study, all MRI scans were reassessed by an experienced radiologist (T.V.), who was blinded to the patients' histopathological and clinical treatment outcomes. The following parameters were recorded: T-stage, tumor morphology (presence of SRCC pattern), N-stage (assessing border contour and signal intensity characteristics of malignant lymph nodes) [Citation11,Citation12], extramural vascular tumor invasion [Citation13,Citation14], extramesorectal nodal distribution [Citation15], and the presence of middle rectal vessels [Citation16].
Therapeutic regimen
Radiotherapy was planned according to standard Norwegian Gastrointestinal Cancer Group guidelines for preoperative treatment of locally advanced or recurrent rectal cancer. Macroscopic tumor (including any extramesorectal nodal disease), as delineated by the diagnostic MRI, was defined as gross target volume (GTV), with subclinical disease (including lymph nodes at risk) defined as the clinical target volume (CTV). The GTV with an additional 0.5 to 1.5 cm margin received a total dose of 50 Gy, with the CTV receiving 46 Gy, both schedules fractionated in daily 2 Gy fractions. Four of the six patients received concomitant, radiosensitizing chemotherapy (which was not routinely given until December 2003) consisting of daily 5-fluorouracil (bolus injection of 400 mg/m2) followed by calcium folinate injection (100 mg) on days 1–2, for three cycles. Surgery was performed 4–6 weeks after completion of preoperative radiotherapy, following repeat clinical examination, endoscopy review (for the patients with primary cancer), chest x-ray, liver ultrasound, and pelvic MRI. MRI identification of a >1 mm margin between tumor tissue and adjacent structures was considered sufficient to avoid resection of the adjacent specific organ. The definitive surgical procedures were a planned low anterior resection, abdominoperineal resection, or pelvic exenteration, all with complete rectum excision (for the primary rectal cancers). For several of the cases, the surgery was a joint venture by gastrointestinal surgeons, urologists, and plastic surgeons.
Histopathology evaluation and assessment of treatment response
Resected primary tumor specimens were prepared according to validated protocols [Citation9,Citation10], as the specimens were opened from the resection edge to 2 cm above and below the identifiable tumor, formalin-fixed, and cut with 5 mm slice thickness transversely. Large-mount preparations were made of the tumor slices to enable examination of the maximum depth of penetration and assessment of the circumferential resection margin. Histopathological characteristics were recorded according to standard reporting proforma, including pathologic tumor stage after radiation (ypT), tumor morphology, circumferential and distal resection margins, pathologic nodal stage after radiation (ypN), and whether extramural vascular tumor infiltration was present [Citation14]. For the resected recurrent tumor specimen, including inguinal lymph nodes, morphological features, soft tissue infiltration, and surgical margins were correspondingly recorded. All assessments were performed by the hospital's specialist gastrointestinal cancer pathologists and, for this study, reassessed by an experienced pathologist (K.K.G.).
At the retrospective review, two distinctly different responses were noted; either the complete absence of residual tumor cells but with the presence of mucin pools diffusely infiltrating the bowel wall, mesorectum, or adjacent pelvic structures (ypT0 response) or, alternatively, residual diffuse infiltration of tumor cells into the mentioned anatomic structures. In this study, patients with the former histopathological outcome were defined as good-responders, whereas the latter patient group was noted as poor-responders.
Postoperative evaluation and follow-up
Patient follow-up was undertaken at the Department of Surgical Oncology. Standard review procedures included clinical examination, blood tests, chest x-ray, and CT scanning of the abdomen and pelvis, at 3-months intervals for the first year and every 6–9 months thereafter. In this study, the documentation of the last follow-up date for the patients with no evidence of disease or the date of diagnosis of disease progression or recurrence for the patients who developed disseminated disease has been used to define postoperative clinical outcome.
Results
Patient clinical characteristics ()
Table I. Clinical patient characteristics
The study cohort consisted of five men and one woman with a median age of 65 years (range 52–75 years). Five patients presented with primary cancer, and one patient had locally recurrent disease.
Good-Responder #3 had undergone complete rectum excision of a low rectal cancer at a local hospital 11 months prior to the referral to our institution. Histopathology of the primary surgical specimen, reassessed by a pathologist at our institution, showed rectal SRCC (pT3N2 disease). Resection margins were apparently free; however, following the primary surgery, the patient had noted a persistent perineal wound, which was confirmed to represent recurrent SRCC. Biopsy of an inguinal lymph node confirmed metastasis, but further disease dissemination was not observed.
MRI features prior to preoperative radiotherapy (Table II)
Table II. Findings on diagnostic MRI examinations
At diagnostic MRI examinations, all patients with primary cancer had T4 tumors. One patient was noted to have involvement of the peritoneal reflection, whereas in the remaining four patients, primary tumors extension beyond the mesorectal fascia or peripheral demarcation of the mesorectal compartment was observed. Four of the patients were noted to have extramural vascular tumor invasion.
Of the five patients with primary cancer, two had N1 disease (three involved mesorectal lymph nodes) and three had N2 disease (more than four lymph node metastases). In the latter group, two patients also had lymph nodes with clearly pathological signal on the pelvic walls. Middle rectal vessels, which may provide a route for lateral tumor spread to pelvic sidewall lymph nodes [Citation15], were noted in all three patients who later developed systemic disease and also were the poor-responders.
Good-Responder #3, suffering from locally recurrent cancer, had advanced tumor infiltration within the pelvic cavity and into the pelvic floor and perineum. The observed nodal spread, consisting of one small node on the pelvic sidewall and an inguinal node, was in accordance with the lymphatic drainage of the anatomic site of the recurrent tumor and considered to represent tumor dissemination to primary lymph node stations.
Radiotherapy and preoperative MRI evaluation
All six patients completed their preoperative radiotherapy protocols as scheduled, and at preoperative MRI evaluations, the anatomical tumor extension was essentially unaltered compared to the corresponding MRI scans prior to radiotherapy ( and ). However, of the three patients classified as good-responders, an increase in tumor T2-signal, consistent with higher mucin content of the tumor, was observed at the preoperative MRI evaluations. Of the four primary rectal SRCC with extramural vessel invasion, eradication of intravascular tumor cells was noted in the one patient who achieved complete tumor response.
Two poor-responders who had been noted to have involved pelvic wall lymph nodes on presentation were identified with persistent non-resectable lymph nodes and development of retroperitoneal lymph node metastases, consistent with systemic disease, at preoperative MRI assessment. These patients proceeded to palliative surgical procedures for local symptomatic control.
Histopathological treatment response () and therapy outcome
Table III. Histopathology findings in surgical specimens
As indicated by , at histopathological assessment of the surgical specimens, three patients presented complete absence of residual tumor cells but with the presence of mucin pools. Consequently, these patients had clear resection margins. The remaining three patients displayed diffuse infiltration of abundant residual tumor cells into the anatomic structures that had originally been identified to be involved, although one of these patients had achieved a potential modest treatment response in the form of occasional fibrosis and acellular mucin pools in the primary tumor. Using the pathology staging system for irradiated tissues, all good-responders showed ypT0N0 treatment response, whereas the poor-responders were ypT4N2.
On postoperative follow-up (range 23–52 months), all good-responders have no evidence of recurrent disease. In contrast, the poor-responders developed rapid disease dissemination, with two patients being noted to have retroperitoneal lymph node progression at completion of the preoperative treatment protocol. The third poor-responder was diagnosed with peritoneal carcinomatosis 10 months after the surgery.
Discussion
In this study, treatment responses to preoperative radiotherapy, applied in 2 Gy fractionation doses to 50 Gy, in six patients with primary inoperable, locally advanced primary (T4) or recurrent rectal SRCC were examined. To our knowledge, no study specifically investigating the role of MRI in staging of the rectal SRCC subgroup has been previously reported. In this limited patient population, two distinctly different histological tumor responses were observed; the complete absence of residual tumor (ypT0N0 response) or, in clear contrast, the diffuse infiltration of abundant residual tumor cells throughout the bowel wall, mesorectal compartment, and adjacent pelvic structures. Interestingly, the tumors devoid of radiotherapy response had initially presented as N2 diseases. The patients with complete tumor response have not shown evidence of recurrent disease (postoperative observation period of 23–52 months), whereas the poor-responding patients rapidly developed disseminated disease.
In preoperative radiotherapy of rectal cancer, the histopathological response represents the primary treatment effect [Citation17–19], although the ultimate goal of treatment is an anticipated improvement in survival without local failure. Given that rectal SRCC shows a particular propensity for systemic dissemination by lymphatic routes [Citation6], one can hypothesize that successful local tumor control, including at risk lymph node stations, may reduce the hazard of metastatic disease progression.
Following the observation of tumor responses, we questioned whether identification of disease markers by detailed visualization of the pelvic cavity by MRI potentially might correlate to complete or, alternatively, lack of response to the preoperative radiotherapy protocol. Such information might ultimately be used to select and treat only those patients likely to achieve long-term disease control. In the limited patient population reported here, the three poor-responders presented with N2 disease, possibly suggesting that extensive lymph node dissemination at presentation of rectal SRCC may correlate with lack of radiation response. In non-SRCC rectal adenocarcinoma, the number of involved mesorectal lymph nodes is well recognized to influence and predict outcome [Citation20,Citation21]. It is possible that advanced N-stage rectal cancer at initial patient presentation may represent more intrinsically aggressive tumor biology and, in addition to being extensive disease, may be a surrogate biomarker for clonogenic tumor cell burden.
The presence of involved pelvic sidewall lymph nodes in rectal cancer has been suggested to increase the risk of systemic disease dissemination [Citation16]. In the SRCC population reported here, of three non-responding patients with MRI evidence of middle rectal vessels, two patients had pelvic wall lymph node metastases at diagnosis. These two patients had developed retroperitoneal lymph node progression at preoperative evaluation. Although limited, this data may imply that the simultaneous presence of extensive mesorectal lymph node metastases (N2 disease) and extramesorectal lymph node disease within the pelvic cavity represents an advanced stage of rectal SRCC that cannot be considered curable.
Combined neoadjuvant chemoradiotherapy is known to result in more favorable histological tumor responses than single-modality radiotherapy [Citation1,Citation22], as recently reported also for T4 rectal cancer patients treated at our institution [Citation23]. In the present study, the two patients that had radiotherapy without radiosensitizing chemotherapy completed the preoperative treatment as poor-responders.
From a radiobiological point of view, the two distinctly different histological responses of rectal SRCC to the radiotherapy are intriguing. Review of the literature has not shown specific studies addressing the biological mechanism underpinning this variation in response. Of note, in operable esophageal or esophagogastric junction adenocarcinoma, however, post-radiotherapy survival outcome was significantly better for the SRCC and mucinous tumors than for conventional-type adenocarcinoma [Citation24]. This outcome data strongly suggests that radiotherapy with curative intent may result in complete eradication of tumor cells in selected patient groups with gastrointestinal SRCC entities.
To conclude from this limited number of cases, treatment of locally advanced primary and recurrent SRCC of the rectum with preoperative radiotherapy was associated with significant tumor response and no evidence of recurrent disease in the patients presenting with limited lymph node disease. Despite poorer outcome compared to patients with other rectal adenocarcinomas, our review implies that selected patients with locally advanced growth of rectal SRCC should be offered preoperative radiotherapy with curative intent. We believe this information may be of note for radiotherapy centers that treat rectal cancer.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Valentini V, Beets-Tan R, Borras JM, Krivokapic Z, Leer JW, Pahlman L, . Evidence and research in rectal cancer. Radiother Oncol 2008; 87:449–74.
- Wiig JN, Larsen SG, Dueland S, Giercksky KE. Preoperative irradiation and surgery for local recurrence of rectal and rectosigmoid cancer. Prognostic factors with regard to survival and further local recurrence. Colorectal Dis 2008; 10:48–57.
- Smith N, Brown G. Preoperative staging of rectal cancer. Acta Oncol 2008; 47:20–31.
- Anthony T, George R, Rodriguez-Bigas M, Petrelli NJ. Primary signet-ring cell carcinoma of the colon and rectum. Ann Surg Oncol 1996; 3:344–8.
- Bittorf B, Merkel S, Matzel KE, Wein A, Dimmler A, Hohenberger W. Primary signet-ring cell carcinoma of the colorectum. Langenbecks Arch Surg 2004; 389:178–83.
- Chen JS, Hsieh PS, Hung SY, Tang R, Tsai WS, Changchien CR, . Clinical significance of signet ring cell rectal carcinoma. Int J Colorectal Dis 2004; 19:102–7.
- Kang H, O'Connell JB, Maggard MA, Sack J, Ko CY. A 10-year outcomes evaluation of mucinous and signet-ring cell carcinoma of the colon and rectum. Dis Colon Rectum 2005; 48:1161–8.
- Hamilton SR, Vogelstein B, Kudo S. Carcinoma of the colon and rectum. Hamilton SR, Aaltonen LA. Pathology and genetics of tumours of the digestive system Lyon IARC Press; 2000.105–19.
- MERCURY Study Group. Diagnostic accuracy of preoperative magnetic resonance imaging in predicting curative resection of rectal cancer: Prospective observational study. BMJ 2006; 333:779–
- MERCURY Study Group. Extramural depth of tumor invasion at thin-section MR in patients with rectal cancer: Results of the MERCURY study. Radiology 2007; 243:132–9.
- Brown G, Richards CJ, Bourne MW, Newcombe RG, Radcliffe AG, Dallimore NS, . Morphologic predictors of lymph node status in rectal cancer with use of high-spatial-resolution MR imaging with histopathologic comparison. Radiology 2003; 227:371–7.
- Kim JH, Beets GL, Kim MJ, Kessels AG, Beets-Tan RG. High-resolution MR imaging for nodal staging in rectal cancer: Are there any criteria in addition to size? Eur J Radiol 2004; 52:78–83.
- Brown G, Radcliffe AG, Newcombe RG, Dallimore NS, Bourne MW, Williams GT. Preoperative assessment of prognostic factors in rectal cancer using high-resolution magnetic resonance imaging. Br J Surg 2003; 90:355–64.
- Smith NJ, Barbachano Y, Norman AR, Swift RI, Abulafi AM, Brown G. Prognostic significance of magnetic resonance imaging-detected extramural vascular invasion in rectal cancer. Br J Surg 2008; 95:229–36.
- Koh DM, Brown G, Husband JE. Nodal staging in rectal cancer. Abdom Imaging 2006; 31:652–9.
- Brown G, Kirkham A, Williams GT, Bourne M, Radcliffe AG, Sayman J, . High-resolution MRI of the anatomy important in total mesorectal excision of the rectum. AJR Am J Roentgenol 2004; 182:431–9.
- Bouzourene H, Bosman FT, Seelentag W, Matter M, Coucke P. Importance of tumor regression assessment in predicting the outcome in patients with locally advanced rectal carcinoma who are treated with preoperative radiotherapy. Cancer 2002; 94:1121–30.
- Vecchio FM, Valentini V, Minsky BD, Padula GDA, Venkatraman ES, Balducci M, . The relationship of pathologic tumor regression grade (TRG) and outcomes after preoperative therapy in rectal cancer. Int J Radiat Oncol Biol Phys 2005; 62:752–60.
- Rödel C, Martus P, Papadoupolos T, Fuzesi L, Klimpfinger M, Fietkau R, . Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol 2005; 23:8688–96.
- Moran MR, James EC, Rothenberger DA, Goldberg SM. Prognostic value of positive lymph nodes in rectal cancer. Dis Colon Rectum 1992; 35:579–81.
- Eriksen MT, Wibe A, Haffner J, Wiig JN. Prognostic groups in 1,676 patients with T3 rectal cancer treated without preoperative radiotherapy. Dis Colon Rectum 2007; 50:156–67.
- Capirci C, Valentini V, Cionini L, De Paoli A, Rodel C, Glynne-Jones R, . Prognostic value of pathologic complete response after neoadjuvant therapy in locally advanced rectal cancer: Long-term analysis of 566 ypCR patients. Int J Radiat Oncol Biol Phys 2008; 72:99–107.
- Larsen SG, Wiig JN, Emblemsvaag HL, Groholt KK, Hole KH, Bentsen A, . Extended TME in locally advanced rectal cancer (T4a) and the clinical role of MRI evaluated neo-adjuvant downstaging. Colorectal Dis 2008; Jul 25:Epub ahead of print.
- Chirieac LR, Swisher SG, Correa AM, Ajani JA, Komaki RR, Rashid A, . Signet-ring cell or mucinous histology after preoperative chemoradiation and survival in patients with esophageal or esophagogastric junction adenocarcinoma. Clin Cancer Res 2005; 11:2229–36.
