Duodenal adenocarcinoma is an unusual and aggressive tumor, with an overall 5-year survival rate of about 25% [Citation1].
Very few data on the activity of anticancer agents are available for patients with advanced disease and, to our knowledge, antitumor activity of irinotecan-containing regimens has not been fully explored for this setting of patients.
We report the evidence of a sustained clinical complete tumor response to FOLFIRI combination in a patient with a sporadic, FOLFOX-refractory rapidly growing advanced duodenal adenocarcinoma.
A 45-year-old Caucasian woman underwent pancreato-duodenectomy in December 2006 because of a pT4pN1M0R0 duodenal adenocarcinoma, followed by adjuvant chemotherapy with eight biweekly cycles of 5-fluorouracil/leucovorin/oxaliplatin (FOLFOX-4), and a subsequent 5-FU 200 mg/m2/die continuous-infusion concomitant with radiation therapy.
In October 2007, the patient underwent hysterectomy, bilateral salpingo-oophorectomy and omentectomy, with histological confirmation of ovarian metastases and evidence of peritoneal carcinomatosis. A close “wait and see” strategy was shared with the patient.
In July 2008, a colonoscopy, performed because of melaena and abdominal pain, showed an ab-estrinseco tumor infiltration of the sigma wall, histologically typified as adenocarcinoma.
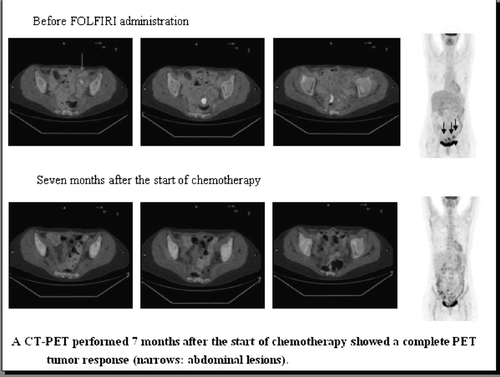
A subsequent FDG-PET-CT scan showed pathological glucose uptake in two peritoneal lesions and in a para-aortic abdominal lymph node (), as well as non-significant uptake (SUV bw max = 1.9) of a sub-centimetric lung lesion.
From July 2008 to February 2009, the patient was treated with a second-line chemotherapy regimen with 5-fluorouracil/leucovorin/irinotecan (FOLFIRI); cetuximab was not added due to the presence of K-ras mutation (codon 13 gly-->asp). FDG-PET-CT performed three, five and seven months after the start of chemotherapy showed a sustained total disappearance of the known abdominal uptake areas, confirmed by the low-dose CT images of PET-CT, without change of the lung uptake area (). During chemotherapy, an early clinical improvement occurred and was maintained over time.
At present, eight months after the start of FOLFIRI, the patient is asymptomatic and without clinical evidence of tumor.
Few data of chemotherapy in patients with advanced small-bowel adenocarcinoma (SBA) are available.
Conflicting data on the activity of different chemotherapy combination arise from retrospective studies [Citation2,Citation3]: an analysis of 217 patients reported benefits of unspecified chemotherapy regimens given to 48 patients as a palliative treatment for advanced SBA, while Jigyasu et al. reported just one partial response among 14 patients treated with a 5-FU-based chemotherapy.
A prospective phase II trial reported a 50% response rate in 30 patients with advanced disease treated with capecitabine plus oxaliplatin [Citation4].
Data regarding CPT-11 in SBA are sporadic and mostly as second-line treatment. In the study by Suenaga seven patients were treated with bi-weekly CPT-11 at 150 mg/m2 and just three stable-disease occurrences were observed, without any partial responses [Citation5].
Here we report a case of a complete and sustained FOLFIRI-induced tumor response in a patient with a rapidly-growing advanced duodenal adenocarcinoma refractory to FOLFOX. Our observation suggests that small bowel adenocarcinomas refractory to oxaliplatin could be highly sensitive to irinotecan-containing chemotherapy combinations.
References
- Sohn TA, Lillemoe KD, Cameron JL, Pitt HA, Kaufman HS, Hruban RH, . Adenocarcinoma of the duodenum: Factors influencing long-term survival. J Gastrointest Surg 1998; 2:79–87.
- Dabaja BS, Suki D, Pro B, Bonnen M, Ajani J. Adenocarcinoma of the small bowel. Presentation, prognostic factors and outcome of 217 patients. Cancer 2004; 101:518.
- Jigyasu D, Bedikian AY, Stroehlein JR. Chemotherapy for primary adenocarcinoma of duodenum. Am J Surg 1984; 53:23–5.
- Overman MJ, Varadhachari GR, Kopetz S, . Phase II study of capecitabine and oxaliplatin for advanced adenocarcinoma of the small bowel and ampulla di Vater. J Clin Oncol 2009; Feb 23:[Epub ahead of print]
- Suenaga M, Mizunuma N, Chin K, . Chemotherapy for small bowel adenocarcinoma at a single institution. Surg Today 2009; 39:27–31.