To the Editor
Malignant peritoneal mesothelioma (MPeM) is a rare disease with dismal prognosis and limited treatment experiences. In Europe the yearly incidence is about one to two cases per million, accounting for 25–33% of all mesotheliomas.
Because of non-specific symptoms, MPeM is often diagnosed late. Symptoms include abdominal pain, distension and ascites.
In the most recent data from Australia, where incidence of malignant mesothelioma is highest in the world, the average survival for MPeM is reported to be five months, with a wide range of 0 to 220 months [Citation1].
Most data on treatment modalities is obtained by single institution experience. Dedicated groups offer to carefully selected patients cytoreductive surgery, peritonectomy, followed by peritoneal chemotherapy perfusion. Median survival rates of 28–35 months are to be expected in experienced hands [Citation2,Citation3]. Some morbidity and mortality (2%) is associated with this combined surgical approach. Treatment failures occur mainly with incomplete cytoreduction. In analogy to pleural mesothelioma, chemotherapy alone in MPeM results in response rates of 10–15% with single agents and of 25% with combined treatment (i.e., platin and pemetrexed) respectively. Results from the international expanded access program (EAP) using pemetrexed alone or in combination with a platinum agent enrolling 109 patients with MPeM 1-year overall survival rate is reported to be 47%. In this cohort, 45% of patients were chemo-naïve, 53% had previous treatment and 2% were unknown in this respect [Citation4].
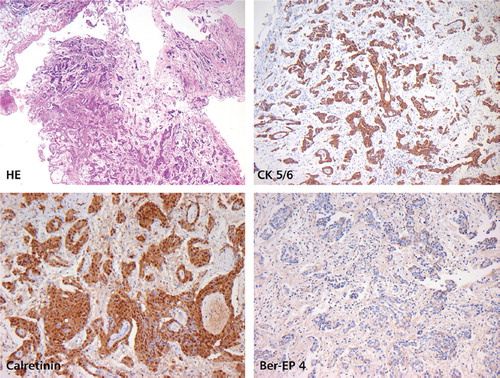
We report on a 48-year-old patient, who was diagnosed with MPeM five years earlier. At diagnosis he had bulky disease and ascites (). Laparoscopically a biopsy was taken from the peritoneum. Histology work up revealed fatty tissue diffusely infiltrated by a mainly papillary, partially tubular and partially solid proliferation of epitheloid rather monomorphic cells with round slightly vesicular nuclei and prominent nucleoli with no production of mucin. These cells immunohistochemically stained strongly positive for Calretinin and CD5/6. Tumor cells stained negative for Ber-EP4, CEA and TTF-1. The proliferation rate was low (10%) as assessed in a MIB-1 stain. The surrounding stroma showed slight myxoid changes. An epitheloid malignant mesothelioma of the peritoneum was diagnosed.
Figure 1. Contrast-enhanced axial CT image during the portal venous phase demonstrates the MPeM (arrow) located in the left upper quadrant of the abdomen. Please note the broad attachment of the tumor to the stomach.
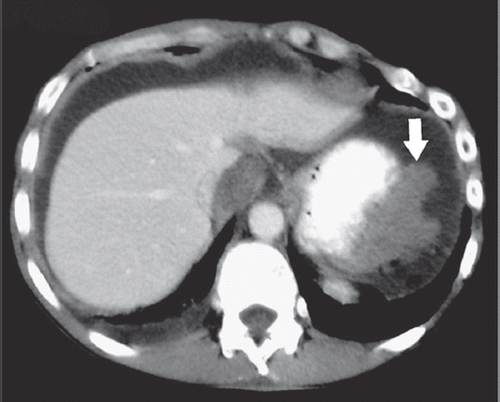
The epitheloid variant of a peritoneal mesothelioma is associated with a better prognosis. True stromal invasion separates benign from malignant mesothelial proliferation. Histology was reviewed and confirmed by the Institute for Surgical Pathology of the University Hospital Zürich (). MPeM in this patient was thought to be a sequela of abdominal irradiation 30 years earlier for Hodgkin’s disease.
Figure 2. Contrast-enhanced axial CT image during the portal venous phase shows complete regression of the MPeM in the left upper quadrant of the abdomen consistent with adequate response to chemotherapy.
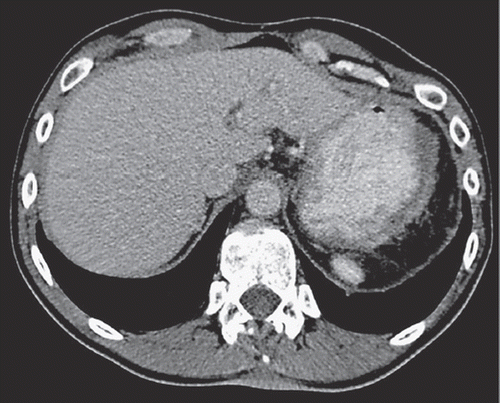
According to promising results in pleural mesothelioma [Citation5] and having access to an EAP we treated the patient with pemetrexed and carboplatin three weekly for one year, followed by pemetrexed monotherapy thereafter. An almost complete response on CT scan was documented 2¾ years after first administration of chemotherapy (). Maintenance therapy with pemetrexed is still ongoing five years after initial diagnosis. Patient’s quality of life is without any restrictions.
Figure 3. Malignant peritoneal mesothelioma, epitheloid variant. Tumor architecture is partially papillary, partially tubular with solid components (H&E). Positive staining for CK5/6 and Calretinin. Negative staining for Ber-EP4. Low proliferation rate (MIB-1 staining not shown).
Our observation is supported by results of a phase II trial on pemetrexed plus gemcitabine as first-line chemotherapy for patients with MPeM [Citation6]. Results seem promising with a median time to disease progression of 10.4 months and a median overall survival of 26.8 months (95%CI of 11.7 months to not reached, 50% censored). In this phase II trial, median number of cycles were 6 (range 1–10). Fifty percent of patients (n = 10) completed planned therapy. Main reasons for discontinuation were toxicity in 25% (n = 5) and progressive disease in 15% (n = 3). Small patient numbers (n = 20) and a wide confidence-interval in this trial limit its evidence. The histological spectrum of MPeM’s, the resulting biology and prognosis has be taken into account, when novel treatment results are interpreted.
Our patient has a favourable MPeM histology which may explain prolonged progression free survival.
CT scan and FDG-PET are not reliable in detecting non-bulky tumor growth in the peritoneum. To define a complete remission or to detect early recurrence of MPeM’s other diagnostic tools are warranted. Soluble mesothelin-related peptides have been proposed as blood-based biomarkers in this respect. High false positive rates are a limitation of the method [Citation7,Citation8]. Therefore appropriate surveillance of residual disease and rules for stopping a maintenance therapy are lacking. As cure of a MPeM with chemotherapy alone is expected to be very unlikely, a well tolerated maintenance therapy might be justified.
Our observation and two other case reports [Citation9,Citation10] let us hypothesize that maintenance therapy with pemetrexed may improve outcome in a subgroup of patients with favourable MPeM`s initially responsive to the treatment.
References
- Clements M, Berry G, Shi J, Ware S, Yates D, Johnson A. Projected mesothelioma incidence in men in New South Wales. Occup Environ Med 2007; 64:747–52.
- Chua TC, Yan TD, Morris DL. Peritoneal mesothelioma: Current understanding and management. Can J Surg 2009; 52:59–64.
- Hassan R, Alexander R, Antman K, Boffetta P, Churg A, Coit D, . Current treatment options and biology of peritoneal mesothelioma: Meeting summary of the first NIH peritoneal mesothelioma conference. Ann Oncol 2006; 17:1615–9.
- Carteni G, Manegold C, Martin Garcia G, Siena S, Zielinski CC, Amadori D, . Malignant peritoneal mesothelioma – Results from the International Expanded Access Program using pemetrexed alone or in combination with a platinum agent. Lung Cancer 2009; 64:211–8.
- Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P, . Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003; 21:2636–44.
- Simon GR, Verschraegen CF, Jänne PA, Langer CJ, Dowlati A, Gadgeel SM, . Pemetrexed plus gemcitabine as first-line chemotherapy for patients with peritoneal mesothelioma: Final report of a phase II trial. J Clin Oncol 2008; 26:3567–72.
- Scherpereel A, Grigoriu B, Conti M, Gey T, Grégoire M, Copin MC, . Soluble mesothelin-related peptides in the diagnosis of malignant pleural mesothelioma. Am J Respir Crit Care Med 2006; 173:1155–60.
- Park EK, Sandrini A, Yates D, Creaney J, Robinson BW, Thomas PS, . Soluble mesothelin-realted protein in an Asbetos-exposed population. Am J Respir Crit Care Med 2008; 178:832–7.
- Fasola G, Puglisi F, Follador A, Aita M, Di Terlizzi S, Belvedere O. Dramatic tumour response to pemetrexed single-agent in an elderly patient with malignant peritoneal mesothelioma: A case report. BMC Cancer 2006; 6:289–
- Miliauskas S, Zemaitis M, Pranys D. Malignant pleural and peritoneal mesothelioma: Incidental diagnosis and excellent treatment results. J Thor Oncol 2009; 4:435–6.
