Abstract
Background. Although some randomized controlled trials had compared the anti-CD20 monoclonal antibody rituximab plus chemotherapy (R-chemo) to chemotherapy alone for B-cell non-Hodgkin's lymphoma, the curative effects of R-chemo were still controversial. A systematic review and meta-analysis was performed to examine the efficacy of using R-chemo compared with the identical chemotherapy alone in the patients with B-cell non-Hodgkin's lymphoma. Material and methods. Medical databases and conference proceedings were searched for randomized controlled trials which compared R-chemo with chemotherapy alone in patients with newly diagnosed or relapsed B-cell non-Hodgkin's lymphoma. Endpoints were overall survival, overall response, disease control, and adverse events. Results. Twelve eligible trials were identified, reporting outcomes of 4 996 patients. Fixed-effects analysis showed overall survival to be superior for R-chemo-treated patients (relative risks [RR], 1.09; 95%confidence interval [CI], 1.06–1.12, p <0.00001). Superiority was also observed for the patients receiving R-chemo with respect to overall response (RR, 1.17; 95%CI, 1.10–1.25, p <0.00001), complete response (RR, 1.52; 95%CI, 1.27–1.82, p <0.00001), and disease control (RR, 1.36; 95%CI, 1.26–1.46, p <0.00001). R-chemo improved overall survival, overall response and disease control in patients with diffuse large B-cell lymphoma (RR, 1.11, 95%CI: 1.06–1.16, p <0.0001; RR, 1.09, 95%CI: 1.01–1.19, p = 0.03 and RR, 2.00, 95%CI: 1.59–2.53, p< 0.00001, respectively) and follicular lymphoma (RR, 1.08, 95%CI: 1.04–1.12, p <0.0001; RR, 1.19, 95%CI: 1.07–1.33, p =0.001 and RR, 2.58, 95%CI: 1.61–4.12, p <0.0001, respectively). Meanwhile, R-chemo improved overall response in patients with mantle cell lymphoma (RR, 1.22, 95%CI: 1.07–1.40, p =0.004). Conclusion. R-chemo is superior to chemotherapy alone in patients with B-cell non-Hodgkin's lymphoma, especially for diffuse large B-cell lymphoma and follicular lymphoma.
Non-Hodgkin's lymphoma is the most common hematologic cancer in adults, with the incidence of more than 66 000 cases anticipated in the United states in 2008 [Citation1]. Approximately 85% of non-Hodgkin's lymphomas in adults are of B-cell origin. Some B-cell non-Hodgkin's lymphomas are indolent, or slow growing, yet incurable. In contrast, others are aggressive or highly aggressive, and may be rapidly fatal, yet are often curable [Citation2]. Diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL) and mantle cell lymphoma (MCL) are the main subtypes of B-cell lymphoma. Diffuse large B-cell lymphoma, with shorter overall survival and progression-free survival, is the most common subtype of aggressive B-cell lymphoma. Although patients with diffuse large B-cell lymphoma are potentially curable when treated with standard regimen: cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) and the 5-year overall survival rate is higher than 40% [Citation3], 30% of patients are insensitive to treatment or will be relapsed in a short period [Citation4]. Radiotherapy is the standard treatment of follicular lymphoma with early stage (Ann Arbor stage I or II), However, less than 15-20% of patients with follicular lym-phoma are diagnosed at an early stage of the disease, and only half of the patients experience long-term disease-free survival after radiotherapy, the vast majority of patients with follicular lymphoma are at advanced stage disease (Ann Arbor stage III or IV) and cannot be cured with conventional therapy. The prognosis was good for patients with follicular lym-phoma, for whom the median survival is 8–10 years [Citation5]. However, many patients with mantle cell lym-phoma (60–70%) have more aggressive disease, and for them, the progression-free survival is very short and the median overall survival is 3–4 years [Citation6]. Recently, there has been a revolution in the treatment of B-cell non-Hodgkin's lymphomas, owing largely to the availability of therapeutic monoclonal antibodies, especially for rituximab. Rituximab is a chimeric monoclonal antibody composed of murine variable regions from the anti-CD20 antibody 2B8 that are linked to a human Fc component directed against CD20 on B cells, which kills tumor cells through complement-dependent cytotoxicity, antibody-dependent cellular cytotoxicity, and induction of apoptosis [Citation7,Citation8]. Rituximab was initially approved by the FDA largely on the basis of McLaughlin's study involving 166 patients with follicular or low-grade non-Hodgkin's lymphoma. The overall response rate was 48%, with 6% of the patients having complete remission [Citation9]. Meanwhile, other study also confirmed the better curative effects of monotherapy of ritux-imab in the treatment of patients with relapsing or refractory aggressive B-cell lymphoma [Citation10]. Although some randomized controlled trials compared the rituximab plus chemotherapy (R-chemo) to chemotherapy alone for B-cell lymphoma, the curative effects of R-chemo is still controversial. Schulz et al. [Citation11] performed a metaanalysis of seven randomized controlled trials, which compared rituximab plus chemotherapy (R-chemo) with chemotherapy alone for 1 943 patients with advanced indolent lymphoma or mantle cell lymphoma, showed that patients treated with R-chemo had better overall response, disease control (describing different endpoints for treatment outcome, including event-free survival, time to treatment failure, progression-free survival and time to progression) and overall survival. However, the meta-analysis did not compare R-chemo with chemotherapy alone for patients with DLBCL, which is the most common subtype of aggressive B-cell lymphoma. We performed this study to incorporate other studies including patients with DLBCL to investigate the effects and toxicities of immuno-chemotherapy with rituximab for B-cell non-Hodg-kin's lymphoma.
Methods
Literature search
To ensure retrieval of all relevant trials, we used a broad search strategy in which key words and text words related to lymphoma and rituximab were combined with a validated methodological filter, as described by Dickersin et al. [Citation12]. The following keywords were used: ‘‘rituximab” and “Lymphoma, B-Cell”. We used this strategy to search a variety of electronic databases, including the PubMed database (1966 to July 2008), the Cochrane Controlled Trials Register (issue 3, 2008), EMBASE (1974 to July 2008) and Chinese Biomedical database (1978 to July 2008). We also manually searched the conference proceedings of the American Society of Hematology (ASH), the American Society of Clinical Oncology (ASCO), and the European Society of Medical Oncology (ESMO) from the year of 1995 to 2008 for relevant clinical trials. Reference lists from studies selected for this review, and from other published systematic reviews and practice guidelines were also hand-searched. Some pharmaceutical companies identified as being active in the field were asked to provide unpublished data or studies.
Inclusion criteria
We included in this analysis only randomized controlled trials that enrolled patients who were older than 18 years and who had histologically proven B-cell non-Hodgkin's lymphoma, regardless of stage of disease and previous therapy received, and that compared R-chemo with the same chemotherapy alone. Trials were included regardless of publication status, date of publication, and language. We excluded ongoing studies, interim analyses, non-randomized studies, and studies with ten or fewer patients per study arm. Studies on the patients with human immunodeficiency virus or primary central nervous system lymphoma were excluded.
Study selection, quality assessment and data extraction
Two reviewers (Guanghui Gao and Jingwei Jiang) independently screened the titles and abstracts of all studies identified in the literature search to verify compliance with the inclusion. When this information was unsatisfactory, full-text was retrieved and inclusion criteria were applied. Disagreements between the two reviewers were resolved by consensus involving a third reviewer (Xiaohua Liang). The same reviewers who screened the studies independently performed data extraction and quality assessment of all included articles. The methodological quality of the studies included in the meta-analysis was scored using the Jadad composite scale. This is a five-point scale, and one point was given when one quality criterion was met [Citation13]. All included studies, regardless of whether they are published or not, were assessed for internal validity parameters, with particular emphasis on randomization, masking of patients and clinicians, concealment of allocation, documentation of dropouts and withdrawals, and intent-to-treat analysis. Each criterion was rated as yes, no or not applicable. Follow-up was arbitrarily defined as complete if trial enrolment was closed, all patients were accounted, and less than 10% were lost in follow-up.
Outcome measures
The primary outcome was overall survival (as defined in [Citation14]). Secondary outcomes included progression-free survival, event-free survival, time to treatment failure, time to progression and adverse events.
Data analysis and statistical methods
A fixed-effect model was assumed in all meta-analyses. The heterogeneity of the studies was tested and a p-value less than 0.1 were defined as hetero-genous. In the presence of statistically significant heterogeneity a random-effect model was applied. For binary data, the relative risks (RR) was used for overall survival, overall response and disease control; the odds ratio (OR) was used for treatment toxicity. A funnel plot was generated or a linear regression test [Citation15] was performed to examine whether there was publication bias. Potential causes of heterogeneity were explored by performing sensitivity analyses to evaluate effects of lymphoma subtype, previous treatment, stage, study duration, study quality, the source of the data, or the influence of single large studies on the effectiveness of rituximab treatment. Particular emphasis was placed on the evaluation of additional side effects of R-chemo in comparison to chemotherapy alone. Side effects were defined as any adverse event occurring during treatment, including death (according to World Health Organization grading).
Analyses were performed using the computer program Review Manager (RevMan; version 4.2.10 for Windows. Statistical tests for heterogeneity were one-sided; statistical tests for effect estimates and publication bias were two-sided.
Results
Identification of studies
The flow chart of our study is shown in . The literature search identified 2 525 trials, of which 45 were considered potentially relevant. The remaining 45 articles were selected for analysis and were evaluated in more detail. Of these, 33 were excluded for the following reasons: three trials did not use identical chemotherapy in the control arm; four articles described patients with human immunodeficiency virus or primary central nervous system lymphoma; nine trials were not randomized; 17 studies using rituximab as monotherapy, maintenance therapy, consolidation therapy, in combination with radiotherapy, or as sequential treatment. The remaining 12 randomized controlled trials (), which involved 4 996 adult patients, met all the inclusion and exclusion criteria and were included in the systematic review and meta-analysis.
Figure 1. Flow chart showing the progress of trials through the review (RCT: randomized controlled trials).
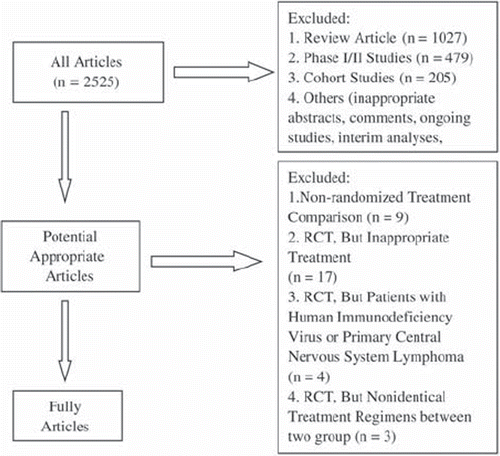
Table I. Summary of trials included in the meta-analysis.*
Two trials [Citation17,Citation26] included relapsed or refractory patients with follicular or mantle cell lym-phoma. The other 10 trials included untreated patients with diffuse large B-cell lymphoma [Citation16,Citation18,Citation22,Citation24], follicular lymphoma [Citation19,Citation20,Citation23,Citation25] or mantle cell lymphoma [Citation19]. The chemotherapy regimens used included: cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP); cyclophosphamide, mitoxantrone, vincristine and prednisone (CNOP); cyclophosphamide, vincristine and prednisone (CVP); fludarabine, cyclophosph-amide and mitoxantrone (FCM); mitoxantrone, chlorambucile and prednisolone (MCP); cyclo-phosphamide, doxorubicin, vincristine, etoposide and prednisone (CHOEP); methotrexate, doxoru-bicin, cyclophosphamide, vincristine, prednisone and bleomycin (MACOP-B); prednisone, mitoxan-trone, cyclophosphamide, etoposide, bleomycin and vincristine (PMitCEBO). All trials compared one of these regimens in combination with rituximab (indicated as R-chemo) with the chemotherapy regimen alone (). The regimen of one trial [Citation27] was CHOP every 14 days (CHOP-14) and the regimen of other seventrials [Citation16,Citation18,Citation20–22,Citation24,Citation26] were CHOP every 21 days (CHOP-21). In one trial [Citation25], patients were also randomly assigned to a third group to assess treatment with rituximab alone; those patients were not included in this meta-analysis. In two trials of R-CHOP versus CHOP, patients who were younger than 60 years [Citation20] or younger than 65 years [Citation21] and in remission were eligible for a second random assignment to adjuvant treatment with high-dose chemotherapy followed by either blood stem cell transplantation or inter-feron alpha maintenance; patients in remission who were 60 years or older [Citation20] or 65 years or older [Citation21] received interferon alpha maintenance. Three studies, one of the FCM regimen combined with rituximab versus FCM [Citation17] and the other two trials of R-CHOP versus CHOP [Citation18,Citation26] offered patients in remission a second random assignment to either rituximab maintenance or observation. All the trials that offered a second random assignment showed a balanced distribution of the baseline characteristics of the patients included in the initial R-chemo and chemotherapy arms.
Study quality
Quality assessment of the included trials is shown in . All studies were described by the authors as randomized. The method of allocation concealment was not described in three trials [Citation21,Citation22,Citation25]. All studies reported intent-to-treat analyses except for one, which was unclear [25], and few dropouts were described. Ten studies [Citation16–18,Citation20–24,Citation26,Citation27] were published as full-text articles, and two [Citation19,Citation25] were published in abstract form.
Overall survival (11 trials, 4 933 patients)
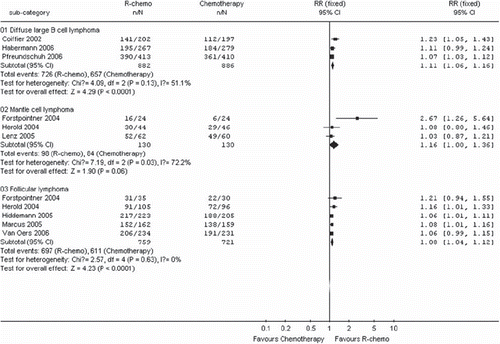
The median observation time ranged from 18 to 42 months and the number of patients for overall survival was calculated by assuming a 2-year overall survival from published studies. Patients in the R-chemo group had a statistical significant better overall survival in the R-chemo group compared with the chemotherapy-alone group (RR, 1.09, 95% CI: 1.06–1.12, p <0.00001, fixed-effects model) (). Sub-group analyses of diffuse large B-cell lymphoma patients and follicular lymphoma patients also revealed that the R-chemo arms had statistical significant higher overall survival than the chemotherapy-only arms (RR, 1.11,95% CI: 1.06–1.16, p <0.0001 andRR, 1.08,95% CI: 1.04–1.12, p <0.0001,respectively). However, there was only a trend toward improving overall survival in the subgroup of patients with mantle cell lymphoma who had received R-chemo, the numbers of patients (n=260) were too small to yield statistical significance (RR, 1.16; 95%CI: 1.00-1.36; p = 0.06) ().
Figure 2. Meta-analysis of overall survival among patients receiving rituximab with chemotherapy (R-chemo) or chemotherapy alone. n = number of events; N = number of patients; 95% CI = 95% confidence interval; RR = relative risks; The diamond shows the 95% confidence intervals for the pooled relative risks. Values greater than 1.0 indicate relative risks that favor R-chemo.
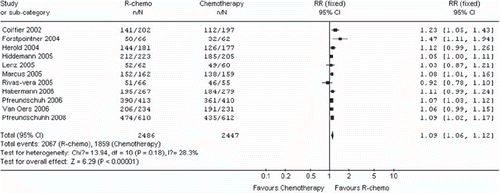
Figure 3. Overall survival for the subgroups of patients with diffuse large B-cell lymphoma, mantle cell lymphoma or follicular lymphoma receiving rituximab with chemotherapy (R-chemo) or chemotherapy alone. n = number of events; N = number of patients; 95% CI = 95% confidence interval; RR = relative risks; The diamond shows the 95% confidence intervals for the pooled relative risks. Values greater than 1.0 indicate relative risks that favor R-chemo.
Overall response (11 trials, 4 141 patients)
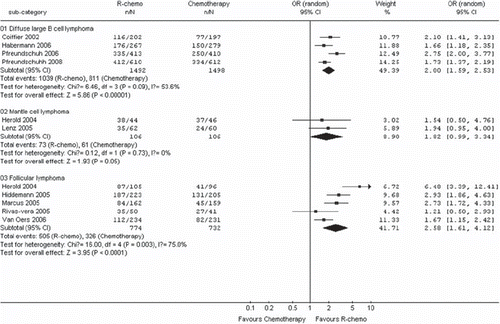
Patients in the R-chemo group had a statistically significantly better response rate compared to patients in the chemotherapy-alone group (RR, 1.17, 95% CI: 1.10–1.25, p <0.00001, random-effects model) (). The rate of complete response was statistically significantly higher in patients treated with R-chemo compared to patients treated with chemotherapy alone (RR, 1.52, 95% CI: 1.27-1.82, p <0.00001, random-effects model) (). The improved response rates have not changed in all subtype of B-cell lymphoma ().
Figure 4. Meta-analysis of overall response rate for patients receiving rituximab with chemotherapy (R-chemo) or chemotherapy alone. n = number of events; N = number of patients; 95% CI = 95% confidence interval; RR ˆrelative risks; The diamond shows the 95% confidence intervals for the pooled relative risks. Values greater than 1.0 indicate relative risks that favor R-chemo.
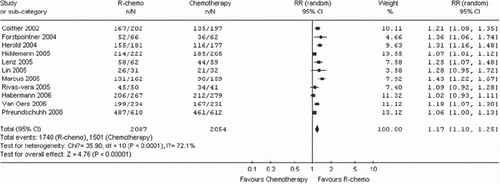
Figure 5. Meta-analysis of complete response for patients receiving rituximab with chemotherapy (R-chemo) or chemotherapy alone. n = number of events; N = number of patients; 95% CI = 95% confidence interval; RR = relative risks; The diamond shows the 95% confidence intervals for the pooled relative risks. Values greater than 1.0 indicate relative risks that favor R-chemo.
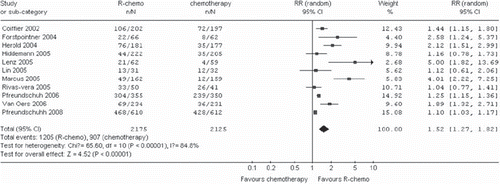
Figure 6. Overall response rate for the subgroups of patients with diffuse large B-cell lymphoma, mantle cell lymphoma or follicular lymphoma receiving rituximab with chemotherapy (R-chemo) or chemotherapy alone. n ˆnumber of events; N ˆnumber of patients; 95% CI = 95% confidence interval; RR = relative risks; The diamond shows the 95% confidence intervals for the pooled relative risks. Values greater than 1.0 indicate relative risks that favor R-chemo.
Disease control (11 trials, 4 903 patients)
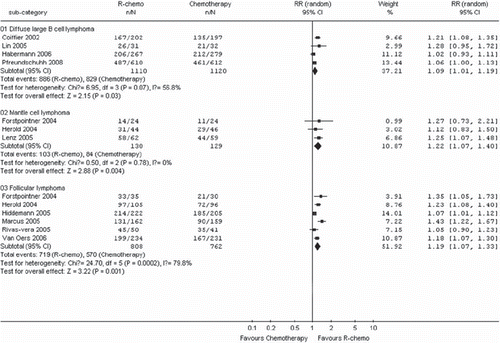
The eleven trials [Citation16–21,Citation23–27] included in the meta-analysis described different endpoints for treatment outcome, including event-free survival, time to treatment failure, progression-free survival, and time to progression. The number of patients for disease control was calculated by assuming a 2-year disease control rate from published studies. R-chemo was statistically significantly superior to chemotherapy alone (RR, 1.36, 95% CI: 1.26 to 1.46, p <.00001, random-effects model) (). R-chemo was also statistically significantly superior to chemotherapy alone when subgroups of diffuse large B-cell lymphoma and follicular lymphoma were analyzed (). However, there was only a trend toward improving disease control in the subgroup of patients with mantle cell lymphoma who had received R-chemo, the numbers of patients (n = 212) were too small to yield statistical significance (RR, 1.82, 95% CI: 0.99-3.34, p=0.05) ().
Figure 7. Meta-analysis of disease control for all patients receiving rituximab with chemotherapy (R-chemo) or chemotherapy alone. Disease control is shown as the relative risks (RR) for a disease event (progression, relapse, death). n = number of events; N = number of patients; 95% CI = 95% confidence interval; RR = relative risks; The diamond shows the 95% confidence intervals for the pooled relative risks. Values greater than 1.0 indicate relative risks that favor R-chemo.
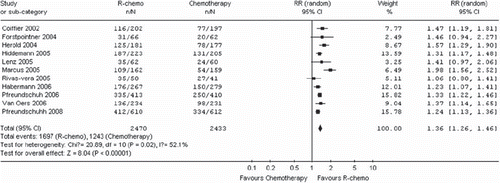
Figure 8. Disease control for the subgroups of patients with diffuse large B-cell lymphoma, mantle cell lymphoma or follicular lymphoma receiving rituximab with chemotherapy (R-chemo) or chemotherapy alone. Disease control is shown as the relative risks (RR) for a disease event (progression, relapse, death). n ˆnumber of events; N ˆnumber of patients; 95% CI =95% confidence interval; RR = relative risks; The diamond shows the 95% confidence intervals for the pooled relative risks. Values greater than 1.0 indicate relative risks that favor R-chemo.
Adverse events
Adverse events were not uniformly described. Four trials [Citation16,Citation17,Citation20,Citation21] analyzed toxicity over treatment cycles rather than recording absolute numbers of adverse events. Therefore, we performed a metaanal-ysis of adverse events among the other eight trials that reported absolute numbers of adverse events [Citation18,Citation19,Citation22–27]. The most often reported grade 3 and 4 adverse events were hematologic toxicity (i.e., leu-kocytopenia, thrombocytopenia, or granulocytope-nia), fever, and infection. The odds ratio (OR) for developing fever or leukocytopenia was statistically significantly higher in patients treated with R-chemo than in patients treated with chemotherapy alone (OR, 4.18, 95% CI: 1.55–11.28, p <0.001 and OR, 1.32, 95% CI: 1.10–1.58, p =0.003, respectively). There was no difference between two groups with respect to the risk of treat-related deaths (OR, 1.09, 95% CI: 0.80-1.49, p =0.58) ().
Table II. Summary of toxicity meta-analyses of treatment for B-cell lymphoma among patients receiving rituximab with chemotherapy (R-chemo) or chemotherapy alone*.
Discussion
The studies were relatively heterogeneous with respect to patient population with different subtypes of B-cell lymphoma, treatment regimen, and treatment duration. Given this clinical heterogeneity our decision to meta-analyze for overall response, disease control, overall survival, and key adverse event rates could be questioned. We believe that meta-analysis was appropriate and helpful in this case as it illustrated a significant and consistent relative benefit of R-chemo as compared to chemotherapy alone. There was no statistically significant difference between the two groups in disease control and overall survival with mantle cell lymphoma. There were several possible explanations for the reasons: only three randomized controlled studies [Citation17,Citation19,Citation21] included patients with mantle cell lymphoma; the numbers of patients were too small to yield statistical significance; 2-year follow-up time for calculation in this analysis may be too short to show the advantage of rituximab; three RCTs included untreated, relapsed or refractory patients with mantle cell lymphoma, which may be heterogeneous. There is a clear need for more prospective randomized trials for untreated patients and patients with relapsed or refractory mantle cell lymphoma.
The studies included in this analysis offered a variety of chemotherapy regimens, such as CHOP, FCM, CNOP, CHOEP and so on. Lacking of enough “head to head” trials comparing rituximab plus different chemotherapy regimens, with the currently available data, we cannot comment about which chemotherapy regimen is the best to combine with rituximab. The NHL-B2 trial [Citation28] had confirmed that CHOP-14 arms had statistically significantly longer event-free survival and overall survival than the CHOP-21 arms, but whether the R-CHOP-14 had an advantage of curative effects comparing with R-CHOP-21 was uncertain. The number of cycles scheduled applied ranged from four to eight. Ghielmini et al. had reported a significant event-free survival and response duration with the prolonged treatment with rituximab in patients with follicular lymphoma compared with the standard weekly × 4 schedule [Citation29]. With the currently available data, we cannot comment about the optimal number of cycles needed to treat the patients with B-cell lymphoma, but six-cycle treatment plus rituximab (R-CHOP-14) would be a proper choice for elder patients with diffuse large B-cell lymphoma [Citation27]. Otherwise, the impact of maintenance treatment with rituximab, which was not evaluated in this analysis, was one of the most important open questions for patients with B-cell non-Hodgkin's lymphoma.
Our meta-analysis was based on trials reported in the literature or at major international conferences. As such, our review has a number of important limitations. First, our review is vulnerable to publication bias. We attempted to minimize the impact of publication bias by including presentations from ASH, ASCO and ESMO, but it is possible that negative trials of rituximab may not have been published or presented at these venues. As well, our analyses were limited to the data presented and/ or shared by authors of the source studies. In some cases we had incomplete information. Our metaanalysis was based on aggregate study and substudy data, not on individual patient data. As a result, we had some limitation in the time-to-event analyses we were able to perform. As well, it was not possible to explore whether patient factors contributed to the statistical heterogeneity we observed in curative effects. Finally, the quality of a meta-analysis is always subject to the quality of included studies. All of the 12 trials included in this meta-analysis were moderate to large RCTs that employed intent-to-treat analyses. However, masking of allocation was inconsistently reported and no study was blinded. The absence of blinding likely had minimal impact on outcomes such as death or disease progression, but may have affected adverse event rates. As well, there was relatively little information on the methods and analyses of the two unpublished RCTs making detailed quality assessments challenging. Despite the limitations of our study, we believe that it makes an important contribution to the B-cell lymphoma field. Prior to our meta-analysis, only one study [Citation11], had reported a significant overall survival benefit with the addition of rituximab to standard treatment of B-cell lymphoma. Importantly, our analysis confirms that R-CHOP should be the standard treatment for patients with untreated diffuse large B-cell lymphoma.
Conclusion
This meta-analysis demonstrated that, in patients with B-cell lymphoma, R-chemo is superior to chemotherapy alone with respect to overall response, disease control and overall survival. Therefore, concomitant treatment with rituximab and standard chemotherapy regimens should be considered the standard of care for patients with B-cell lymphomas, especially for diffuse large B-cell lymphoma and follicular lymphoma.
Acknowledgements
The work is supported by the Scientific Research Foundation of Huashan Hospital, Fudan University, Shanghai, China.
Declaration of interest: The authors indicated no potential conflicts of interest.
References
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, . Cancer statistics, 2008. CA Cancer J Clin; 2008;58:71–96.
- Armitage JO, Weisenburger DD. New approach to classifying non-Hodgkin's lymphomas: Clinical features of the major histologic subtypes. J Clin Oncol 1998;16:2780–95.
- Sehn LH, Berry B, Chhanabhai M, Fitzgerald C, Gill K, Hoskins P, . The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood 2007;109:1857–61.
- Kenkre VP, Smith SM. Management of relapsed diffuse large B-cell lymphoma. Curr Oncol Rep 2008;10:393–03.
- Bendandi M. Aming at a curative strategy for follicular lymphoma. CA Cancer J Clin 2008;58:305–17.
- Andersen NS, Jensen MK, de Nully Brown P, Geisler CH. A Danish population-based analysis of 105 mantle cell lym-phoma patients: Incidences, clinical features, response, survival and prognostic factors. Eur J Cancer 2002;38:401–8.
- Shan D, Ledbetter JA, Press OW. Apoptosis of malignant human B cells by ligation of CD20 with monoclonal antibodies. Blood 1998;191:1644–52.
- Demidem A, Lam T, Alas S, Hariharan K, Hanna N, Bonavida B, . Chimeric anti-CD20 (IDEC-C2B8) monoclonal antibody sensitizes a B cell lymphoma cell line to cell killing by cytotoxic drugs. Cancer Biother Radio-pharm 1997;12:177–86.
- McLaughlin P, Grillo-Lopez AJ, Link BK, Levy R, Czuczman MS, Williams ME, . Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: Half of patients respond to a four-dose treatment program. J Clin Oncol 1998;16:2825–33.
- Coiffier B, Haioun C, Ketterer N, Engert A, Tilly H, Ma D, . Rituximab (anti-CD20 monoclonal anti-body) for the treatment of patients with relapsing or refractory aggressive lymphoma: A multicenter phase II study. Blood 1998;92:1927–32.
- Schulz H, Bohlius JF, Trelle S, Skoetz N, Reiser M, Kober T, . Immunochemotherapy with rituximab and overall survival in patients with indolent or mantle cell lymphoma: A systematic review and meta-analysis. J Natl Cancer Inst 2007;99:706–14.
- Dickersin K, Scherer R, Lefebvre C. Identifying relevant studies for systematic reviews. BMJ 1994;309:1286–91.
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, . Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials 1996;17:1–12.
- Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horing SJ, . Revised response criteria for malignant lymphoma. J Clin Oncol 2007;25:579–86.
- Egger M, Davey SG, Schneider M, Minder C. Bias in metaanalysis detected by a simple, graphical test. BMJ 1997;315:629–34.
- Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, . CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med 2002;346:235–2.
- Forstpointner R, Dreyling M, Repp R, Hermann S, Hanel A, Metzner B, . The addition of rituximab to a combination of fludarabine, cyclophosphamide, mitoxantrone (FCM) sig-nificantly increases the response rate and prolongs survival as compared with FCM alone in patients with relapsed and refractory follicular and mantle cell lymphomas: Results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood 2004;104:3064–71.
- Habermann TM, Weller EA, Morrison VA, Gascoyne RD, Cassileth PA, Cohn JB, . Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol 2006 24:121–7.
- Herold M, Pasold R, Srock S, Neser S, Niederwieser D, Neubauer A, . Results of a prospective randomised open label phase III study comparing rituximab plus mitoxan-trone, chlorambucil, prednisolone chemotherapy (R-MCP) versus MCP alone in untreated advanced indolent non-Hodgkin's lymphoma (NHL) and mantle-cell-lymphoma (MCL). Blood 2004;104:abstract584.
- Hiddemann W, Kneba M, Dreyling M, Schmitz N, Lengfelder E, Schmits R, . Frontline therapy with rituximab added to the combinat ion of cylophosphamide, doxorubicin, vinc-ristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lym-phoma compared with therapy with CHOP alone: Results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood 2005;106:3725–32.
- Lenz G, Dreyling M, Hoster E, Wörmann B, Dührsen U, Metzner B, . Immunochemotherapy with rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisone significantly improves response and time to treatment failure, but not long-term outcome in patients with previously untreated mantle cell lymphoma: Results of a prospective randomized trial of the German Low Grade Lymphoma Study Group (GLSG). J Clin Oncol 2005;23:1984–92.
- Lin TY, Zhang HY, Huang Y, Guan ZZ, Shen T, Shi YK . Comparison between R-CHOP regimen and CHOP regimen in treating naive diffuse large B-cell lymphoma in China-a multi-center randomized trail. Ai Zheng 2005;24:1421–6.
- Marcus R, Imrie K, Belch A, Cunningham D, Flores E, Catalano J, . CVP chemotherapy plus rituximab compared with CVP as first-line treatment for advanced follicular lymphoma. Blood 2005;105:1417–23.
- Pfreundschuh M, Trümper L, Osterborg A, Pettengell R, Trneny M, Imrie K, . CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lym-phoma: A randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol 2006;7:379–91.
- Rivas-Vera S, Baez E, Sobrevilla-Calvo P, Baltazar S, Tripp F, Vela J, . Is first line single agent rituximab the best treatment for indolent non-Hodgkin's Lymphoma? Update of a multicentric study comparing rituximab vs CNOP vs rituximab plus CNOP. Blood 2005;106:abstract2431.
- van Oers MH, Klasa R, Marcus RE, Wolf M, Kimby E, Gascoyne RD, . Rituximab maintenance improves clinical outcome of relapsed/ref ractory follicular non-Hodgkin lym-phoma in patients both with and without rituximab during induction: Results of a prospective randomized phase 3 inter-group trial. Blood 2006;108:3295–301.
- Pfreundschuh M, Schubert J, Ziepert M, Schmits R, Mohren M, Lengfelder E, . Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: A randomised controlled trial (RICOVER-60). Lancet Oncol 2008;9:105–16.
- Pfreundschuh M, Trümper L, Kloess M, Schmits R, Feller AC, Rübe C, . Two-weekly or 3-weekly CHOP chemotherapy with or without etoposide for the treatment of elderly patients with aggressive lymphomas: Results of the NHL-B2 trial of the DSHNHL. Blood 2004;104:634–1.
- Ghielmini M, Schmitz SF, Cogliatti SB, Pichert G, Hum-merjohann J, Waltzer U, . Prolonged treatment with rituximab in patients with follicular lymphoma significantly increases event-free survival and response duration compared with the standard weekly x 4 schedule. Blood 2004;103:4416–23.
