Abstract
Purpose. Oncolytic adenovirus such as ZD55 has become a promising anticancer agent for its efficient tumor-targeted replication and lysis capability. Armed with therapeutic gene IL-24 to generate a novel oncolytic adenovirus ZD55-IL-24, the antitumor efficiency of ZD55 is greatly increased. To explore the clinical application of ZD55-IL-24 in cancer therapy, the combination of gene-virotherapy (ZD55-IL-24) with chemotherapy was performed in this paper. Methods. The effect of this gene-virotherapy with chemotherapy on cell proliferation was determined by MTT assay in four types of cancer cell lines and one human normal cell line. Real-time PCR was performed to detect the replication of ZD55-IL-24 when adriamycin (ADM) or cisplatin (DDP) was administrated. The changes in caspase pathway were analyzed by Western blot. We further identify the combinational therapy in Balb/c nude mice with NCI-H460 xenograft. Results. ADM and DDP enhanced cell killing/inhibiting effects of ZD55-IL-24 in all the tumor cell lines, while no overlapping toxicity was observed in the normal liver cell line L-02. These chemo-agents inhibited the propagation of ZD55-IL-24 in NCI-H460 cells, but did not influence the expression of IL-24. Consistent with the results in vitro, the tumor growth of co-administration group was remarkably delayed, compared with single treatment groups (p<0.05). Conclusion. ZD55-IL-24 combined with ADM demonstrates improved killing effects against lung tumor xenograft.
Oncolytic adenoviruses, which are engineered to replicate selectively in tumor cells, have been applied as a novel and promising remedy for cancer therapy [Citation1]. ONYX-015isoneofthe examples of E1B-55kD-deleted oncolytic adenoviruses. Recent work revealed that the major determinant of ONYX-015 tumor-selective replication in tumor cells is due to the loss of E1B-55K-mediated late viral RNA export rather than p53 degradation [Citation2]. The ONYX-015 combined with chemotherapy has demonstrated potent clinical activity [Citation3]. To get even better anti-cancer efficiency, an E1B-55kD-deleted adenovirus (ZD55) similar to but much different from ONYX-015 was constructed. The major difference between ZD55 and ONYX-015 is that a cloning site was introduced into ZD55 for inserting antitumor genes. The combination strategy of oncolytic virus therapy and gene therapy is called ‘‘Targeting Gene-Viro-therapy’’ [Citation4].
In this study, IL-24/mda-7, recognized as a ‘‘magic bullet’’ for treating diverse cancers [Citation5], is used as the therapeutic gene. Numerous studies have shown that IL-24 promotes tumor-specific apoptosis through both secretory and non-secretory pathways in diverse human cancers, while sparing normal cells [Citation6,Citation7]. Additionally, the antitumor effects of IL-24 are cell-type dependent. For example, IL-24 negatively regulates the β-catenin and PI3K signaling pathways in breast and lung tumor cells [Citation8], activates RNA-dependent protein kinase (PKR) in lung cancer cells [Citation9], mediates selective apoptosis in human melanoma cells by means of p38 MAPK [Citation10], kills ovarian cancer cells through activation of the Fas-FasL signaling pathway [Citation11], and kills pancreatic cancer cells by inhibition of the Wnt/PI3K pathways [Citation12].
The most desirable character of IL-24 is its ‘‘bystander’’ antitumor activity, along with inhibiting angiogenesis and stimulating an antitumor immune response. An injection of IL-24 armed oncolytic adenovirus into human breast cancer xenografts in athymic nude mice completely eradicated not only the primary tumor but also distant tumors [Citation13]. These properties make IL-24 an excellent therapeutical gene for our targeted genevirotherapy.
Although ZD55-IL-24 demonstrated potent cancerinhibiting effects in vitro and in vivo when a colorectal carcinoma xenograft was used [Citation14], the combination of ZD55-IL-24 and conventional chemotherapy may exhibit better curative effects.
The purpose of our study was to assess the combined effect of genevirotherapy and chemotherapy. Our results confirmed that both adriamycin (ADM) and cisplatin (DDP) in vitro and AMD in human lung cancer xenograft enhance the apoptosis induced by ZD55-IL-24.
Materials and methods
Cell lines and culture
The human lung cancer cell lines A549, NCI-H460, human cervical epithelial carcinoma cell HeLa, fibrosarcoma cell HT-1080 and normal human liver cell L-02 were purchased from Shanghai Cell Collection (Chinese Academy of Sciences, China). NCI-H460 cells were maintained in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS) (GIBCO-BRL, Gaithersburg, MD), and the other cells were cultured in Dulbecco's modified essential medium (DMEM) with 10% FBS at 37°C in a 95% air–5% CO2 humidified incubator.
Real-time quantitative polymerase chain reaction (qPCR)
NCI-H460 cells were seeded in 6-well plates at 106 each well and were infected at 10 multiplicity of infection (MOI) with ZD55-IL-24. Twenty-four hours postinfection, the cells were washed with phosphate buffered saline (PBS) three times, and detached by trypsin-EDTANa2. The cell pellet was resuspended in 100 μl of 100 mM Tris-HCl (pH 8.0), and cells were broken by three cycles of freezing and thawing, followed by incubation with 200 μl of ProtK-SDS solution (proteinase K (0.5 mg/ml), 10 mM Tris-HCl (pH 7.5), 0.5% sodium dodecyl sulfate (SDS), 10 mM EDTA (pH 8.0)) for 3 hour at 37°C. DNA was then precipitated by addition of 1/10 volume of 3 M sodium acetate (pH 5.2) and a 2.5 volume of 95% ethanol, rinsed with 70% ethanol, dried, and resuspended in 100 μl of water and then was diluted at 1:50 as template [Citation15]. Quantifications of adenovirus E4 region were performed by qPCR and the specific pairs of primers for E4 region included E4-forward (5′-CTAACCAGCGTAGCC CCGA-3′) and E4-reverse (5′-TGAGCAGCACCT TGCATTTT-3′).
Cell viability assay
Cells were seeded in 96-well plates and treated with ZD55-IL-24/ZD55-EGFP, adriamycin (doxorubicin) (Alexis, Switzerland), cisplatin (Alexis, Switzerland) alone or combination of viruses with chemical medicines. At the indicated times, the medium was removed and 10 μl 0.5 mg/ml 3-(4, 5-dimethylthia-zol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) was added to each well. Cells were incubated at 37°C for four hours. Then, the MTT was removed and 100 )μl/well DMSO was added. Absorption was read on a microplate reader with 450 nm excitation and 590 nm emission (Fluorescence Reader, TE-CAN AUSTRIA G.M.B.H.) for the detection of the cell viability.
Apoptotic cell staining
NCI-H460cells were seeded in 6-well plates. After treatment with viruses and chemical drugs for 72 hours, cells were incubated with Hoechst 33342 (1μg/ml) for 30 minutes, and the apoptotic morphological changes of tumor cells are observed under a fluorescence microscope immediately.
Western blot analysis
Equal amounts of total proteins were separated on 10% or 12% polyacrylamide gels and transferred onto 0.45 [im nitrocellulose membrane (Millipore) in a buffer containing 25 mM Tris-HCl (pH 8.3)/ 192 mM glycine/20% methanol and blocked with 5% non-fat milk. Membranes were incubated with primary antibodies, followed by the addition of anti-rabbit IR Dye 700 or anti-mouse IR Dye 800 (Rockland Inc., from Lorne Laboratories, Reading, UK) and fluorescent signal was revealed by using the Odyssey Infrared Imaging System (LI-COR, Lincoln, NE). The primary antibodies were purchased from Santa Cruz Biotechnology (anti-PARP, anti-PKR and anti-E1A, 1:1000), GenHunter (anti-IL-24, 1:1000) and Jing-Mei (anti-β actin, 1:5000).
In vivo tumor xenografts
Animal welfare and experimental procedures in these experiments were carried out strictly in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996). Female BALB/c nude mice (4–6 weeks of age) were obtained from the Shanghai Experimental Animal Center of the Chinese Academy of Science (Shanghai, China). Aliquots of NCI-H460 cells (1 × 107) in 100 )μl of PBS were subcutaneously inoculated into the right flank of nude mice. Tumors were allowed to grow until they reached approximately 150 mm3 calculated from the following equation: tumor volume V (mm3) = 1/2 × length (mm) × width (mm)2. Mice were divided randomly into four groups (five mice per group) and treated by intratumor injection of ZD55-IL-24 at 1 × 109 plague-forming units (pfu) per animal or PBS as a control. Doxorubicin was injected into the intraperitoneum for the same time at a dose of 4 mg/kg body weight. The tumor was monitored by measuring the tumor size weekly.
Immunohistochemistry (IHC) and TdT-mediated dUTP-biotin nick end-labeling (TUNEL) assay
Deparaffinized tumor sections were treated with rabbit monoclonal anti-E1A antibody (diluted 1:1000). After incubation with an anti-rabbit secondary antibody, expression of E1 A in cells was detected with diaminobenzidine (DAB; Sigma, St. Louis, MO) by an avidin-biotin reaction ABC kit (Vector Laboratories, Burlingame, CA). Tissue sections stained without primary antibody were served as negative control. The sections were then counterstained with hematoxylin. TdT-mediated dUTP-biotin nick end-labeling (TUNEL) assay was performed by the protocol of Promega.
Statistical analysis
All values are presented as mean with standard deviation. Statistical significance was determined by the Student's t-test. All p-values less than 0.05 were considered significant.
Results
Effects of chemo-agents on the propagation of ZD55-IL-24
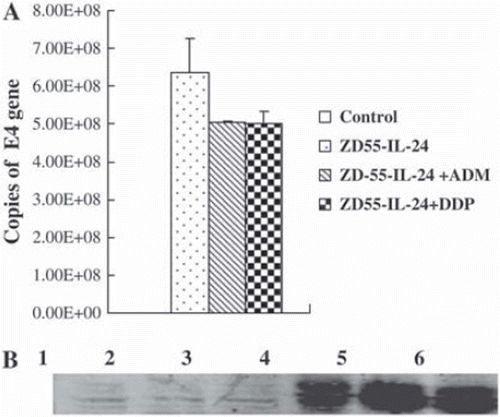
Although the combination of ONYX-015 and standard chemotherapy was done a decade ago and demonstrated exciting antitumor activity [Citation16], the reasons for the enhanced antitumor effect when combining oncolytic adenovirus with chemo-agents are not well known. Moreover, the sequence in which viruses and chemotherapy are administrated may affect the effect of tumor cell death [Citation17]. To address these problems, quantitive PCR was performed to measure the replication of ZD55-IL-24 by calculating copies of adenoviral E4 region when cells were treated by ZD55-IL-24 and chemoagents. All cells were harvested 24 hours after virus infection.
As shown in , ADM or DDP showed a little inhibition effects on the replication of ZD55-IL-24, but did not affect the expression of IL-24.
Figure 1. Effects of doxorubicin and cisplatin on the propagation of ZD55-IL-24.
(A) Quantitative PCR to detect the replication of ZD55-IL-24 in NCI-H460 cells.
(B) Analysis of IL-24 expression by Western blotting with the presence of doxorubicin and cisplatin. NCI-H460 cells were infected with ZD55-IL-24 at an MOI of 10 pfu/cell, and IL-24 protein was analyzed 48 hours later in cell lysates. A total of 20 μg protein was loaded on 10% polyacrylamide gels and separated. Lane 1 control, lane 2 ADM (80 nM), lane 3 DDP (200 nM), lane 4 ZD55-IL24, lane 5 ZD55-IL24+ADM (80 nM), lane 6 ZD55-IL24 + DDP (200 nM).
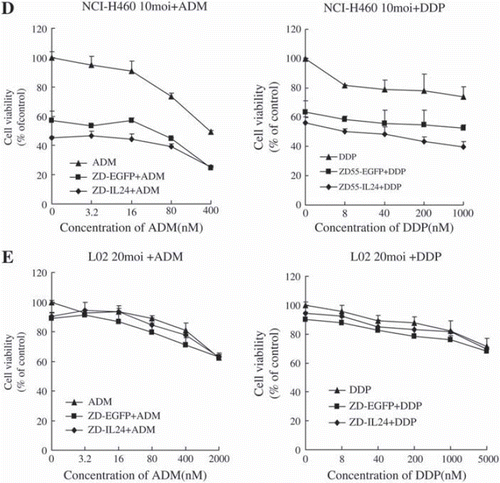
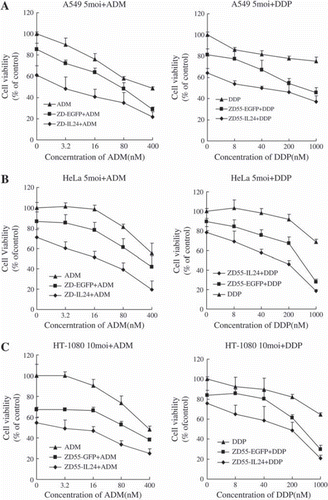
Enhanced efficacy of ZD55-IL-24 combined with ADM or DDP against cancer cells
To determine whether ADM or DDP could enhance the cell killing effect of ZD55-IL-24, NCI-H460 cells were treated with ZD55-IL-24 plus drugs at various concentrations. As shown in , after treatment with an indicated MOI of ZD55-IL-24/ ZD55-EGFP plus ADM with varying concentrations (3.2 ng-400 ng/ml) or DDP (8 ng-1 000 ng/ml), cell viability was reduced in all combination treatments when compared with single agent treatments or untreated cells (p<0.05). The combinational treatments showed additive therapeutic activity in reducing the cancer cells. Normal cell line L-02 was examined as the control and no overlapping cytotoxicity was observed even when treated with viruses at 20 moi (p>0.05).
Figure 2. Cell killing effect of ZD55-IL-24 was enhanced when combined with Doxorubicin and cisplatin against cancer cell lines but not normal cells.
Cells were infected with 5 moi (A549 and HeLa), 10 moi (NCI-H460 and HT-1080), or 20 moi (L-02). Cell viability was measured with MTT assay 72 hours after treatment.
ADM/DDP enhanced ZD55-IL-24-mediated apoptotic signaling
Based on the experiments above, NCI-H460 cells were analyzed for apoptotic morphological changes by Hoechst 33342 staining after treatment with ZD55-IL-24 or in combination with ADM/DDP. As demonstrated in , most cancer cells underwent apoptosis when treated by ZD55-IL-24 in combination with ADM or DDP.
Figure 3. The combination induced much more tumor-specific apoptosis in NCI-H460 cells.
(A) Cell apoptotic staining. After 72 hrs of treatment, NCI-H460 cells were incubated with Hoechst 33342 for 30 minutes, and condensation and fragmentation of nuclei were observed under a fluorescence microscope (arrow).
(B) The cleavage of caspase substrate PARP was assessed. PKR expression was also determined. Lane 1 control, lane 2 ADM (80 nM), lane 3 DDP (200 nM), lane 4 ZD55-IL24, lane 5 ZD55-IL24+ADM (80 nM), lane 6 ZD55-IL24 + DDP (200 nM), lane 7 ZD55-EGFP, lane 8 ZD55-EGFP+ADM (80 nM), lane 9 ZD55-EGFP + DDP (200 nM).
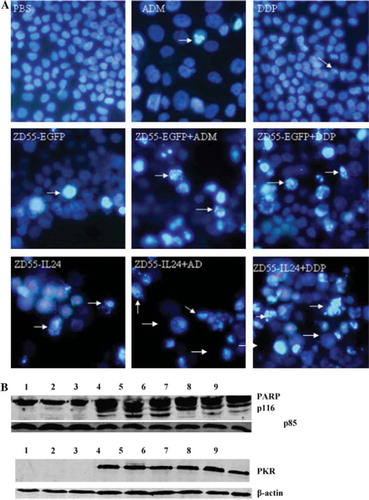
To further determine whether treatment with ZD55-IL24 plus ADM/DDP can affect the cas-pase cascade, we analyzed the substrate of caspases poly (ADP-ribose) polymerase (PARP) by Western blot. The combination of viruses either with ADM or DDP led to a little increase of PARP cleavage, compared to gene viro-therapy or chemotherapy alone. Additionally, ZD55-IL24 is superior to ZD55-EGFP in sensitizing chemotherapy ().
Increased antitumor efficacy of ZD55-IL24 combined with ADM in nude mice
Our in vitro data above proved that ZD55-IL24 combined with ADM led to an additive effect in cancer cell lines. To further evaluate the antitumor activity of ZD55-IL24 alone or in combination with ADM/DDP in lung cancer, NCI-H460 cells (1 × 107 per mouse) were inoculated into the right dorsal flank of nude mice and were left growing for 10 days until tumor volume reached 100–150 mm3. Viruses were administrated intratumorally to respective groups and the doxorubicin was injected intraperitonally at the same time. The same injection was repeated once two days later.
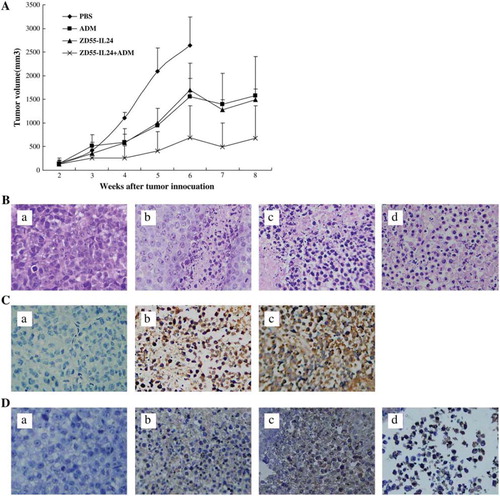
As shown in , tumors treated with viruses or ADM showed significant growth inhibition compared to the PBS-treated group. Moreover, the growth-inhibitory effect of combined treatment with ZD55-IL24 plus ADM was significant greater than that with ZD55-IL24 or ADM alone (pB0.05). There was much more cell death/apoptosis in tumor treated combinationally (). This was due to the replication of ZD55-IL-24 ().
Figure 4. Treatment of NCI-H460 xenograft with ZD55-IL-24 and adriamycin.
Tumor size was measured weekly after injection of ZD55-IL-24 (1 × 109 pfu/tumor) or ZD55-EGFP (1 × 109 pfu/tumor). Adriamycin was injected intraperitoneally (4 mg/kg) at the same time. Each group included at least five mice.
H&E staining of tumors obtained from mice treated with ZD55-IL-24 and/ or adriamycin. The tumor treated with PBS was used as control.
Original magnification ×400. a: PBS as control, b: ADM, c: ZD55-IL24, d: ZD55-IL24+ADM.
Immunohistochemical staining with anti-E1A was performed. Original magnification ×400. Original magnification ×400. a: PBS as control, b: ZD55-IL24, c: ZD55-IL24+ADM.
Apoptotic study in vivo by TUNEL after treatment with viruses. TUNEL assay was performed to detect apoptosis in the tumor section. Original magnification x400. a: PBS as control, b: ADM, c: ZD55-IL24, d: ZD55-IL24+ADM.
Discussion
Even a best designed oncolytic virus can benefit from chemotherapy because of their different mechanisms of cytotoxicity. The combination of oncolytic adenovirus and chemotherapy can be a useful strategy to treat cancer. ONYX-015 and other oncolytic viruses have obtained better clinical effects when combined with chemotherapy (reviewed in [Citation17]), but the mechanisms of combination are not fully understood. The enhanced cell killing effects may due to that the chemo-agents facilitate the spread of oncolylitc adenoviruses releasing from apoptotic cells. Another hypothesis is that chemo-agents can decrease the amount of viruses required for oncolysis [Citation18]. Recently, some groups pointed towards that autophagy besides apoptosis could be very important for adenoviral infection and viron releasing [Citation19].
Many previous studies have proved that chemo-agents can enhance the killing effects of adenovirus in vivo and/or in vitro [Citation3,Citation17]. Interestingly, Valproic Acid (a histone deacetylase inhibitor), despite of its up-regulation of the expressions of coxsackie and adeno-virus receptor (CAR) and viral E1A protein, inhibits adenoviral replication late in the viral life through induction of p21WAF1/CIP1 [Citation20]. Given that the chemo-agents have complex impacts on the cellular signaling pathways and many cellular proteins including topoisomerases and transcription factors [Citation21,Citation22] are needed for efficient replication initiation of adenovirus, it is possible that chemo-agents inhibit the production of adenoviruses indirectly.
Previous studies have shown that Ad-mda7-induced apoptosis in lung cancer cells depends on the interaction between MDA-7 and PKR [Citation9], which is involved in not only antiviral activity, but also cellular functions such as cell proliferation, differentiation and apoptosis [Citation23]. Early studies suggested that PKR acted as a tumor suppressor. For example, transfection of NIH 3T3 cells with a functionally defective mutant PKR led to malignant transformation [Citation24]. However, knockdown of PKR reduces pulmonary metastatic potential of murine melanoma dramatically, which is mediated by the transcription factor NF-κB [Citation25]. In addition, as the expression of PKR and prognosis for certain tumors is concerned, different clinical observations contradicted each other [Citation26]. In our experiments, chemo-agents did not change the expression level of PKR when tumor cells were treated with ZD55-IL-24 () Since PKR facilitates DNA repair and opposes cell apoptosis in response to DNA damaging agents including DDP [Citation27], the role of PKR in the antitumor effect is ambiguous during the combination of ZD55-IL24 and chemotherapy.
The tumor in nude mice did not shrink as we had anticipated (). It was at least partially due to tumors growing too fast and the mean value of tumor volumes was 142.5 mm3 one day before injection. Moreover, the proper proportion between oncolytic adenoviruses and chemo-agents should be calculated.
Although the data presented here showed that ADM or DDP augments tumor killing of ZD55-IL24, they may not be ideal agents for chemo-gene-virotherapy because of their inhibition effect on the replication of adenoviruses. More appropriate chemicals or methods should be found to fit the peculiarities of targeting gene-virotherapry. For example, novel agents and strategies have been developed recently to increase adenoviral replication [Citation28] or expression of ectopic MDA-7 protein in tumor cells [Citation29], resulting in greater growth inhibition of tumor cells.
Theoretically, some cancer cells can be or develop to be omniresistant [Citation30] to all the current cancer treatments, including chemo-agents, oncolytic viruses and IL-24 [Citation31]. Recently, the cancer stem cell (CSC) hypothesis casts some new light on the origination and treatment of cancer. According to the CSC hypothesis, the tumor originates from a subpopulation of cells which have self-renewing ability and stronger resistance to radio- and chemotherapy compared with non-CSC tumor cells. Therefore, eliminating cancer stem cells is greatly required for the clinical anti-tumor treatment. Small molecule inhibitors [Citation32] and immunotoxins [Citation33,Citation34] with CSC specificity are currently intensively researched in the field of targeting cancer stem cell, especially leukemic stem cells (LSC). Still viruses targeted for CSCs are being studied. Recently, Jiang et al. [Citation19] testified the feasibility and the effecitiveness of oncolytic adenovirus Delta-24-RGD in targeting brain tumor stem cells. Due to the difference in tropisms, it is found that the serotype 16 (Ad16) and chimpanzee Ad (CV23) can infect both the CD133 (+) cancer stem cells and the CD 133 (–) cells of the tumor efficiently [Citation35].
In conclusion, we have demonstrated that combinational therapy with ZD55-IL24 and ADM/DDP showed enhanced antitumor effect in lung cancer both in vitro and in vivo. The results provide a basis for further clinical research of ZD55-IL24 plus ADM/DDP against human cancers.
Acknowledgements
This work was supported by the Zhejiang Science and Technology Support Plan (No. 2007C33027). The authors would like to thank Kan Chen for her assistance in flow cytometry analysis. We are grateful to Qiang Zhuang and Yuexiang Yin for the technical assistance in qPCR.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Parato KA, Senger D, Forsyth PA, Bell JC. Recent progress in the battle between oncolytic viruses and tumours Nature Rev 2005;5.
- O’Shea CC, Johnson L, Bagus B, Choi S, Nicholas C, Shen A, . Late viral RNA export, rather than p53 inactivation, determines ONYX-015 tumor selectivity Cancer Cell 2004;6.
- Khuri FR, Nemunaitis J, Ganly I, Arseneau J, Tannock IF, Romel L, . A controlled trial of intratumoral ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer Nature Med 2000;6.
- Liu XY, Gu JF. Targeting gene-virotherapy of cancer Cell Res 2006;16.
- Fisher PB. Is mda-7/IL-24 a ‘‘magic bullet’’ for cancer? Cancer Res 2005;65.
- Lebedeva IV, Su ZZ, Sarkar D, Kitada S, Dent P, Waxman S, . Melanoma differentiation associated gene-7, mda-7/ interleukin-24, induces apoptosis in prostate cancer cells by promoting mitochondrial dysfunction and inducing reactive oxygen species Cancer Res 2003;63.
- Sauane M, Lebedeva IV, Su ZZ, Choo HT, Randolph A, Valerie K, . Melanoma differentiation associated gene-7/ interleukin-24 promotes tumor cell-specific apoptosis through both secretory and nonsecretory pathways Cancer Res 2004;64.
- Mhashilkar AM, Stewart AL, Sieger K, Yang HY, Khimani AH, Ito I, . MDA-7 negatively regulates the beta-catenin and PI3K signaling pathways in breast and lung tumor cells Mol Ther 2003;8.
- Pataer A, Vorburger SA, Chada S, Balachandran S, Barber GN, Roth JA, . Melanoma differentiation-associated gene-7 protein physically associates with the double-stranded RNA-activated protein kinase PKR Mol Ther 2005;11.
- Sarkar D, Su ZZ, Lebedeva IV, Sauane M, Gopalkrishnan RV, Valerie K, . mda-7 (IL-24) Mediates selective apoptosis in human melanoma cells by inducing the coordinated overexpression of the GADD family of genes by means of p38 MAPK Proc Natl Acad Sci USA 2002;99.
- Gopalan B, Litvak A, Sharma S, Mhashilkar AM, Chada S, Ramesh R. Activation of the Fas-FasL signaling pathway by MDA-7/IL-24 kills human ovarian cancer cells Cancer Res 2005;65.
- Chada S, Bocangel D, Ramesh R, Grimm EA, Mumm JB, Mhashilkar AM, . mda-7/IL24 kills pancreatic cancer cells by inhibition of the Wnt/PI3K signaling pathways: Identification of IL-20 receptor-mediated bystander activity against pancreatic cancer Mol Ther 2005;11.
- Sarkar D, Su ZZ, Vozhilla N, Park ES, Gupta P, Fisher PB. Dual cancer-specific targeting strategy cures primary and distant breast carcinomas in nude mice Proc Natl Acad Sci USA 2005;102.
- Zhao L, Gu J, Dong A, Zhang Y, Zhong L, He L, . Potent antitumor activity of oncolytic adenovirus expressing mda-7/ IL-24 for colorectal cancer Human Gene Ther 2005;16.
- Puntel M, Curtin JF, Zirger JM, Muhammad AK, Xiong W, Liu C, . Quantification of high-capacity helper-dependent adenoviral vector genomes in vitro and in vivo, using quantitative TaqMan real-time polymerase chain reaction Human Gene Ther 2006;17.
- Heise C, Sampson-Johannes A, Williams A, McCormick F, Von Hoff DD, Kirn DH. ONYX-015, an E1B gene-attenuated adenovirus, causes tumor-specific cytolysis and anti-tumoral efficacy that can be augmented by standard chemotherapeutic agents Nature Med 1997;3.
- Chu RL, Post DE, Khuri FR, Van Meir EG. Use of replicating oncolytic adenoviruses in combination therapy for cancer Clin Cancer Res 2004;10.
- Nemunaitis J, Khuri F, Ganly I, Arseneau J, Posner M, Vokes E, . Phase II trial of intratumoral administration of ONYX-015, a replication-selective adenovirus, in patients with refractory head and neck cancer J Clin Oncol 2001;19.
- Jiang H, Gomez-Manzano C, Aoki H, Alonso MM, Kondo S, McCormick F, . Examination of the therapeutic potential of Delta-24-RGD in brain tumor stem cells: Role of autophagic cell death J Natl Cancer Inst 2007;99.
- Hoti N, Chowdhury W, Hsieh JT, Sachs MD, Lupoid SE, Rodriguez R. Valproic acid, a histone deacetylase inhibitor, is an antagonist for oncolytic adenoviral gene therapy Mol Ther 2006;14.
- de Jong RN, van der Vliet PC. Mechanism of DNA replication in eukaryotic cells: Cellular host factors stimulating adenovirus DNA replication Gene 1999;236.
- Nagata K, Guggenheimer RA, Hurwitz J. Adenovirus DNA replication in vitro: Synthesis of full-length DNA with purified proteins Proc Natl Acad Sci USA 1983;80.
- Sadler AJ, Williams BR. Structure and function of the protein kinase R Current Topic Microbiol Immunol 2007;316.
- Koromilas AE, Roy S, Barber GN, Katze MG, Sonenberg N. Malignant transformation by a mutant of the IFN-inducible dsRNA-dependent protein kinase Science 1992;257(5077).
- Delgado Andre N, De Lucca FL. Knockdown of PKR expression by RNAi reduces pulmonary metastatic potential of B16-F10 melanoma cells in mice: Possible role of NF-kappaB Cancer Letter 2007;258.
- Garcia MA, Gil J, Ventoso I, Guerra S, Domingo E, Rivas C, . Impact of protein kinase PKR in cell biology: From antiviral to antiproliferative action Microbiol Mol Biol Rev 2006;70.
- Bergeron J, Benlimame N, Zeng-Rong N, Xiao D, Scrivens PJ, Koromilas AE, . Identification of the interferon-inducible double-stranded RNA-dependent protein kinase as a regulator of cellular response to bulky adducts Cancer Res 2000;60.
- Libertini S, Iacuzzo I, Ferraro A, Vitale M, Bifulco M, Fusco A, . Lovastatin enhances the replication of the oncolytic adenovirus dll520 and its antineoplastic activity against anaplastic thyroid carcinoma cells Endocrinology 2007;148.
- Oida Y, Gopalan B, Miyahara R, Inoue S, Branch CD, Mhashilkar AM, . Sulindac enhances adenoviral vector expressing mda-7/IL-24-mediated apoptosis in human lung cancer Mol Cancer Ther 2005;4.
- Vaha-Koskela MJ, Heikkila JE, Hinkkanen AE. Oncolytic viruses in cancer therapy Cancer Letter 2007;254.
- Pataer A, Chada S, Roth JA, Hunt KK, Swisher SG. Development of Ad-mda7/IL-24-resistant lung cancer cell lines Cancer Biol Ther 2008;7.
- Guzman ML, Swiderski CF, Howard DS, Grimes BA, Rossi RM, Szilvassy SJ, . Preferential induction of apoptosis for primary human leukemic stem cells Proc Natl Acad Sci USA 2002;99.
- Feuring-Buske M, Frankel AE, Alexander RL, Gerhard B, Hogge DE. A diphtheria toxin-interleukin 3 fusion protein is cytotoxic to primitive acute myeloid leukemia progenitors but spares normal progenitors Cancer Res 2002;62.
- Du X, Ho M, Pastan I. New immunotoxins targeting CD123, a stem cell antigen on acute myeloid leukemia cells J Immunother 2007;30.
- Skog J, Edlund K, Bergenheim AT, Wadell G. Adenoviruses 16 and CV23 efficiently transduce human low-passage brain tumor and cancer stem cells Mol Ther 2007;15.