To the Editor
We report on a case of penile sarcomatoid carcinoma in a 61-year-old non-smoking man.
History
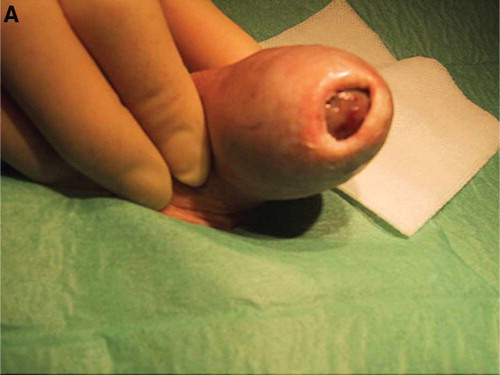
The patient consulted a private urologist because of a pedunculated bleeding tumor of glans penis. The private urologist found a palpable and movable tumor between the prepuce and external meatus ().
Treatment and diagnosis
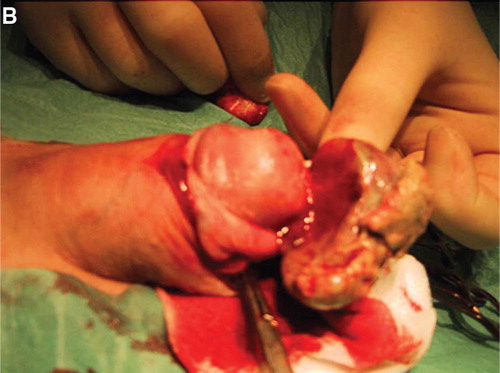
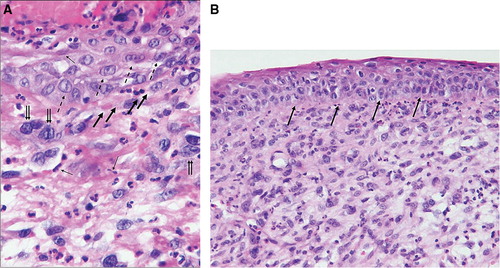
A radical circumcision was performed by the urologist (). The histo-pathological report concluded with invasive squamous cell carcinoma and extensive carcinoma in situ component adjacent to areas with lichen sclerosus and atrophicus. The appearance of the neoplasm varied considerably with squamous cell differentiation at the superficial part and extensive underlying sarcomatoid growth pattern (). Interlacing fascicles with atypical spindle cells were mixed with multinucleated giant cells and areas with pseudovascular spaces mimicking angiosarcoma and malignant fibrous histiocytoma (). These areas were seen in close conjunction with the squamous cell neoplasm (). The polypoid sarcomatoid tumor stained negative for CD31, CD34 and cytokeratins and was positive for vimentin, whereas the epithelial component was cytokeratin positive by immuno-chemistry. The diagnosis sarcomatoid carcinoma was favoured due to the presence of superficial infiltrating squamous cells adjacent to an extensive squamous cell carcinoma in situ component (). Vascular invasion was not seen but the deepest point of tumor invasion was close to the resection margin and a reresection of the prepuce of the penis was consequently performed at The Norwegian Radium Hospital (NRH). The histo-pathological examination did not reveal any cancerous tissue in the reresection specimen, but balanitis xerotica obliterans. Tumor spread was not seen in the subsequent inguinal sentinel lymph node procedure from the left groin.
Figure 2. Hematoxylin-eosin stain section of the lesion. (a) Tumor giant cells are marked with double arrows. Pseudovascular spaces demonstrated with bold arrows. Spindle neoplastic cells exemplified with simple arrows, and purely differentiated squamous epithelial cell carcinoma at the surface are marked with dashed arrows (× 400). (b) Squamous cell carcinoma in situ component as marked with arrows separated from the underlying sarcomatoid tumor tissue (× 200).
The patient was followed at the out-patient department of the NRH and 1.5 year after the primary surgery the patient appeared with a fast growing tumor of glans penis. A palpable enlarged lymph-node in the right groin was resected; histological examination verified a squamous cell carcinoma compatible with metastasis. Sarcomatoid growth pattern was not seen. The patient was operated with partial penile amputation and a surgical margin of 2 cm as well as a radical lymphnode dissection of the right groin. The histopathological report confirmed a squamous cell carcinoma of glans penis. The patient did not receive any other adjuvant oncological treatment.
Discussion
Sarcomatoid carcinoma is an uncommon variant of carcinoma of the penis which in developed countries is estimated to 0.9/100 000. The tumor may present in either a purely sarcomatoid form or most often in association with squamous cell phenotype as in our case (). Sarcomatoid carcinomas of penis are rare occurrences and only a few case reports and two recent series of five and 15 cases exist in the literature [Citation1–8]. The morphological features of the penile tumors are similar to their counterparts in other locations, the majority present with malignant spindle cells with variable patterns with a minor squamous cell component as in our case. Velazquez et al. [Citation1] failed to classify one case in the first biopsy that contained only the superficial squamous cell carcinoma. The sarcomatoid tumor tissue was seen in the deeper part of tumor which was visible in the subsequent resection specimen [Citation1]. Sarcomatoid carcinoma may mimic sarcoma and melanoma which necessitates the need for immunohistological examination. In the present case tumor tissue with squamous cell phenotype was positive for cytokeratins whereas the underlying sarcomatoid tumor mimicking angiosarcoma was negative for cytokeratins, CD31 and CD34 and positive for vimentin. This and the close relationship between the epithelial and the sarcomatoid spindle cell compartment as seen in convinced us that we were dealing with one tumor demonstrating epithelial and sarcomatoid phenotype and our conclusion was sarcomatoid carcinoma.
Sarcomatoid carcinoma may present in either a purely sarcomatoid form or in association with a typical epithelial phenotype as demonstrated in our case. The term carcinosarcoma has been considered synonymous with sarcomatoid carcinoma by some authors [Citation6] but carcinosarcoma shows intimate admixture of carcinomatous and sarcomatous elements. The sarcomatoid component often exhibits heterologeous differentiation toward skeletal muscle or chondroid and osseous tissue which was not seen in our case. There is now a general consensus that the malignant spindle cell compartment is of epithelial origin despite of negative immunostaining for cytokeratins [Citation1,Citation9]. Our findings further support to prefer the term sarcomatoid carcinoma in agreement with Velazquez et al. [Citation1].
Sarcomatoid carcinomas are aggressive and highly malignant tumors. The patient is 4.5 years after the last surgery still cancer free.
Acknowledgements
We are deeply indebted to Professors Juan Rosai from the Instituto Nazionale Per Lo Studio E La Cura Dei Tumori, Milano, Italy, and Robert H. Young from the Department of Pathology, Massachusetts General Hospital, USA, for expert comments in this case of sarcomatoid carcinoma of the penis.
References
- Velazquez EF, Melamed J, Barreto JE, Aguero F, Cubilla AL. Sarcomatoid carcinoma of the penis: A clinicopathological study of 15 cases. Am J Surg Pathol 2005;29:1152–8.
- Cubilla AL, Reuter V, Velazquez E, Piris A, Saito S, Young RH. Histological classification of penile carcinoma and its relation to outcome in 61 patients with primary resection. Int J Surg Pathol 2001;9:111–20.
- Patel B, Hashmat A, Reddy V, Angkustsiri K. Spindle cell carcinoma of penis. Urology 1982;19:93–5.
- Manglani KS, Manaligod JR, Ray B. Spindle cell carcinoma of the glans penis: Alight and electron microscopic study. Cancer 1980;46:2266–72.
- Wood EW, Gardner WA Jr, Brown FM. Spindle cell squamous carcinoma of the penis. J Urol 1972;107:990–1.
- Antonini C, Zucconelli R, Forgiarini O, Chiara A, Briani G, Belmonte P, . Carcinosarcome of penis. Case report and review of the literature. Adv Clin Path 1997;1:281–5.
- Somogyi L, Kalman E. Metaplastic carcinoma of the penis. J Urol 1998;160:2152–3.
- Lont AP, Gallee MPW, Snijders P, Horenblas S. Sarcomatoid squamous cell carcinoma of the penis: A clinical and pathological study of 5 cases. J Urol 2004;172:932–5.
- Choi HR, Sturgis EM, Rosenthal DI, Luna MA, Batsakis JG, El-Naggar AK. Sarcomatoid carcinoma of the head and neck: Molecular evidence for evolution and progression from conventional squamous cell carcinomas. Am J Surg Pathol 2003;27:1216–20.