Abstract
Background. Over a 10-year period from 1990, 445 patients with carcinoma of the oesophagus were admitted to the Norwegian Radium Hospital and 184 of these patients received treatment with curative intent. Even though surgery is the treatment of choice for these patients, many of them suffer from medical conditions that increase the risk for postoperative mortality and morbidity. In a retrospective study, the effect of the curative treatment offered to patients was explored with a particular focus on patients unfit for surgery. Methods. Medical data of the 184 patients treated with curative intent were reviewed and additional clinical information was retrieved from local hospitals and general practitioners. Preoperative radiotherapy followed by surgery was the standard curative treatment for operable patients. Medically inoperable patients were offered radical split-course hyperfractionated radiotherapy followed by a brachytherapy boost. Results. More than 50% (103/184) received non-surgical treatment only. Patients who received radical surgery (n = 81) were younger, had better performance status, less weight loss and dysphagia compared to patients treated with radical radiotherapy (n = 102). One patient received only photodynamic therapy. The 3-year survival was 29% for patients treated with radical surgery, and 8% for patients who received radical radiotherapy. The overall median crude survival for the two groups of patients were 20 months and seven months respectively. Conclusion. The hyperfractionated radiotherapy provided symptom relief without extensive toxicity and with a possibility for cure for patients with oesophageal cancer who are unfit for surgery and chemoradiotherapy. The literature supports the curative potential of high dose accelerated hyperfractionated radiotherapy even though the optimal radiotherapy regimen still needs to be explored.
From 1993 to 1997, the overall 5-year survival of oesophageal cancer in Norway was 6.5% for men and 8.3% for women, while the 5-year survival for localized disease was 11.7% for men and 9.1% for women [Citation1].
For at least 50 years, there has been an ongoing debate on the best curative treatment strategy for these patients, and various treatment combinations have been implemented within different hospitals around the world. Surgery with or without preoperative chemoradiotherapy is traditionally the first treatment of choice, but is associated with high morbidity and mortality. Long-time survival is achieved in a low fraction of patients, 5-year survival being 10–25% overall [Citation2–4], ranging from 80% in stage I to 10% in stage III [Citation5]. Even though radiotherapy alone as a definitive treatment modality does not have a strong position, this treatment has previously been expected to have a potential of providing survival comparable to surgery [Citation6]. To our knowledge, randomized trials comparing radiotherapy alone versus surgery alone have not been performed. The Medical Research Council (MRC) trial of 1986 comparing radical surgery to radical radiotherapy failed owing to poor recruitment and was prematurely closed [Citation7].
On the other hand, over the last decades, different chemotherapy regimens have been explored in randomized trials with a demonstrated limited benefit on median survival from 0 to 5 months [Citation8].
The standard curative treatment regimens at the Norwegian Radium Hospital (NRH) during the 1990s were based on a Nordic study of multimodal treatment performed between 1983 and 1988. In this randomized multicentre study, oesophageal cancer patients were stratified into different groups: A: Operable patients (n = 217) and B: medically inoperable and/or locally advanced disease (n = 100). If randomized to radiotherapy, patients received hyperfractionated treatment: 1.75 Gy × 2 × 10 in two weeks. Prolonged survival was found for operable patients receiving preoperative hyperfractionated radiotherapy ± chemotherapy compared to surgery alone ± chemotherapy [Citation2]. The inoperable patients were randomized to split-course radiotherapy ± chemotherapy. The second course of the radiotherapy (1.75 Gy × 2 × 8) was given after a two-week split. No difference in survival was found between the two groups [Citation9].
Although hyperfractionated accelerated treatment was a rather modern and radiobiologically rational approach at the time, split-course treatment was more commonly used to allow for acute reaction repair as well as for detecting progression of disease or avoiding treating patients who deteriorated during the first course of treatment [Citation10]. Moreover, there were studies observing a trend towards better survival for the hyperfractionated schedule compared to conventional schedule in oesophageal cancer [Citation11]. The results from these studies led to the use of preoperative radiotherapy followed by surgery two to three weeks later as the standard curative treatment for operable patients at the Norwegian Radium Hospital (NRH). For patients who were medically unfit, refused surgery or were borderline with regard to lymph node status, standard curative treatment were radical split-course hyperfractionated radiotherapy followed by an intraluminal brachytherapy boost.
A retrospective study of all patients treated at NRH in the 10-year period from 1990 to 1999 inclusive has been performed. The effect of the curative treatment offered to patients was explored with a particular focus on patients unfit for surgery.
Material and methods
Study design, inclusion and exclusion criteria
Patients newly diagnosed with squamous cell carcinoma (SCC), adenocarcinoma (AC) or other carcinomas of the oesophagus or cardia (ICD-7: 150, ICD-10: C15) referred to the NRH from January 1990 to December 1999 inclusive, were identified from the hospital register (445 patients). The cohort represented unselected patients in a defined geographical area encompassing 35–40% of the Norwegian population [Citation12]. In addition, a few patients were referred to NRH from the neighbouring health region because of individual surgeon preferences. A small fraction of patients might have been treated at their local hospitals due to advanced disease. Medical data was reviewed and additional information for a period of at least three years after treatment or until death was retrieved from local hospitals and general practitioners.
Subgroups of patients, definitions and description of treatment
All patients planned for radical surgery or radical radiotherapy or combinations of the two, i.e., treated with a curative intent, are presented in the current paper. Patients treated without a curative intent will be presented elsewhere.
Since the TNM classification of oesophageal cancer mainly is based on information gained by surgery, the tumors were unfortunately not systematically TNM classified at diagnosis. A retrospective classification of clinical stage based on CT scan descriptions and -- or information gained from surgery, is therefore introduced as follows: localized disease (T1–T2 and N0, M0), locally advanced disease (T3 or regarded as locally advanced without infiltrating surrounding tissue and N0, M0), regional disease (T1–3, N+ and M0), advanced or metastatic disease (T4 or M+) ().
Table I. Patient characteristics
The localization of the oesophageal tumors was divided into three parts based on CT scans or distance from the incisor teeth as assessed during diagnostic endoscopies. The “upper third” reaching from the cricopharyngeal sphincter to 20 cm from the incisor teeth or described as proximal in the patients records, the “middle third” from 20 to 30 cm, or when described as situated around the bifurcation of the trachea, and the “lower third” below 30 cm from the teeth or when described as distal. Tumors involving the cardiac junction, but with an oesophageal origin, were also included. “Others” include patients with overlapping tumors.
The following information was taken from the medical files; performance status according to the WHO scale scored from 0 (able to carry out normal activities) to 4 (bedridden and in need of supportive care), reflux at admission (without information about the underlying cause), and weight loss (in per cent of original weight). In addition, complications defined as treatment related events causing prolonged hospitalisation or supplemental treatment during or after the hospital stay, were counted but not graded: Infections, lung-complications, acute cardiac problems, leakage of the anastomoses, bleeding, fistulas and stenoses. Treatment related mortality was based on the number of patients with perioperative deaths within 30 days or deaths within 30 days after end of radiotherapy and of patients who had a treatment related death in our hospital beyond 30 days after surgery and/or radiation.
Pain was retrospectively classified by the first author (CDA) as follows: “No pain”: the medical history stated that the patients did not experience pain, “Some pain”: information about intermittent pain during meals and/or information about pain without regular pain medication, “Moderate pain”: information about constant, but moderate pain, regular use of non-opioids, “Severe pain”: information about strong pain or use of opioids, “Unknown”: no information in medical files about pain or pain medication.
Guidelines for curative treatment in the study period
Surgery. Surgery was most often being performed as right-sided thoracotomy combined with laparotomy. The approach changed from transthoracic to transhiatal and back to transthoracic over the 10-year period. In the period from October 1990 to June 1993, the routine technique was transhiatal dissection with cervical anastomosis.
Preoperative radiotherapy followed by surgery
Preoperative radiotherapy consisted of a total of 35 Gy given as 1.75 Gy twice daily (6 hours apart), five days a week over two weeks, followed by radical surgery two to three weeks after radiotherapy [Citation2]. The radiotherapy was given as two parallel opposed fields 6 to 8 cm wide with the upper margin at the suprasternal notch and the lower margin at the oesophagogastric junction. The tumor was localised using a barium swallow and conventional 2D simulation.
Radical radiotherapy: External radiotherapy followed by brachytherapy boost
The external radiotherapy consisted of 63 Gy given in two series. The first part was equal to the preoperative regimen and was followed by a second part starting two weeks later. The second part was given with a three-field technique based upon a planning CT scan, 1.75 Gy twice a day, five days a week for eight days, total dose 28 Gy. The clinical target volume (CTV) was defined as the original tumor with 1 cm margin above and below and lateral margins 1 cm outside the oesophageal wall.
Finally, an intraluminal high dose rate brachytherapy boost (8 Gy × 1) was given seven to 13 days after external radiotherapy towards the original intraluminal tumor with 1 cm margin above and below, calculated to a depth of 1 cm from the probe.
In this paper, a total radiotherapy dose above 60 Gy has been regarded as radical radiotherapy.
Other curative treatment
Neither chemotherapy nor photodynamic therapy (PDT) was in regular use.
Follow-up
The patients were seen six weeks after end of treatment, thereafter the follow-up visits were individualized. Both mode and time schedule of evaluation and the description of the treatment results varied between patients, and a systematic review of tumor response was not possible. Evaluation of dysphagia after treatment was based on information about the best swallowing ability ever reported in hospital records or by the local hospital or general practitioners. Patients with post-treatment dysphagia due to progression or relapse of disease were offered an oesophageal stent.
Statistical methods and analyses
Medians with confidence intervals, means and percentages are presented as appropriate. Overall survival was estimated using the Kaplan-Meier method and groups were compared with log-rank tests. The expected important prognostic factors were included in the Cox proportional hazards regression model, and the analysis was done with backward stepwise elimination. Sex, age (continuous), histology (AC vs. SCC), clinical stage, and factors associated with overall survival in the univariate analyses: treatment (radical surgery ± radiotherapy vs radical radiotherapy), performance status (WHO 0 vs. 1 vs. ≥ 2), weight loss (≤ 5%, > 5 and ≤ 10%, > 10%) and tumor length (≤ 5 cm, > 5 cm) were included as possible predictor variables. P values < 0.05 (two-sided) were regarded as statistically significant. The clinical database was established using DataEase®, and the statistical software SPSS® for Windows was used for the statistical analyses.
Results
Patients and symptoms characteristics
Of all patients with a primary oesophageal cancer who had their first admission to NRH in the 10-year study period, 184 patients received treatment with the intention to cure. Patient characteristics at admission, treatment given and symptoms at admission are listed for the total group and divided by treatment (surgery ± radiotherapy vs radical radiotherapy) in and . Patients who received radical surgery were more often men, they were younger, had shorter tumor and more often adenocarcinoma localised in the lower part of the oesophagus compared to patients receiving radical radiotherapy (). In addition, patients receiving radical surgery had better performance status and less weight loss than the radical radiotherapy patients ().
Table II. Symptoms, performance status and use of analgesics at admission
Most patients received more than one treatment-modality as reported in .
One patient received photodynamic treatment (PDT) only. She had slight infiltrative growth, but due to her general and medical condition she was found inoperable. Unfortunately, she had a PDT-related oesophageal necrosis followed by mediastinitis and death, and is not further discussed.
Surgery ± radiotherapy (n = 81)
Seventy-one patients received standard preoperative radiotherapy (35 Gy) followed by radical surgery. One of them had concomitant chemotherapy (Cis-platinum + 5-Fluorouracil). Three of these patients received an additional 28 Gy postoperatively due to microscopic residual tumor.
Seven of the ten patients who received radical surgery without preoperative radiotherapy had adenocarcinoma. Due to residual tumor after surgery, one patient with squamous cell carcinoma had postoperative radiotherapy with concomitant chemotherapy of cisplatinum and 5-fluorouracil (CiFu).
Radical radiotherapy (n = 102)
Of the 44 patients treated with external radiotherapy and intraluminal brachytherapy, 30 received the standard regimen. The other 14 patients received external radiotherapy ranging from 58 to 65 Gy, and the brachytherapy ranging from 6 to 18 Gy. Twenty-one of the 44 patients were initially planned for radical surgery but due to general deterioration (n = 11), alteration of the medical condition (n = 3) or patient preferences (n = 7) the planned treatment was changed.
Fifty-eight patients received ≥ 60 Gy external radiotherapy without brachytherapy boost. Due to general deterioration (n = 30), alteration of the medical condition (n = 4), progression (n = 2), patient preferences (n = 3) or unknown reasons (n = 5), the treatment plan was changed for 44 of 58 patients (surgery or brachytherapy boost was cancelled). They were still regarded as treated with curative intent.
One patient received only 47 Gy as external radiotherapy and treatment was stopped due to heart complications. She died eight days after end of treatment.
Complications
Treatment related mortality occurred in 10 of the 81 surgical patients. All of these had received preoperative radiotherapy. Seven had perioperative deaths within 30 days after surgery. Another two died approximately six weeks after treatment due to multiorgan failure and the last patient was reoperated due to intrathoracic lymph leakage and died of respiratory failure four months after radical surgery without leaving the postoperative ward. One of the patients who received radical radiotherapy without surgery died within 30 days after end of treatment.
Postoperative complications were found in 45 patients, 21 of these had more than one. The complications found were: infections (n = 20), pulmonary problems (n = 20), cardiac problems (n = 10), anastomosis leakage (n = 6), bleeding (n = 3), stenoses (n = 19) and fistulas (n = 3).
Twenty-one of the 102 patients who received radical radiotherapy had complications as follows; infections (n = 7), stenoses (n = 13) and fistulas (n = 2). One patient had two complications.
Follow-up
Dysphagia
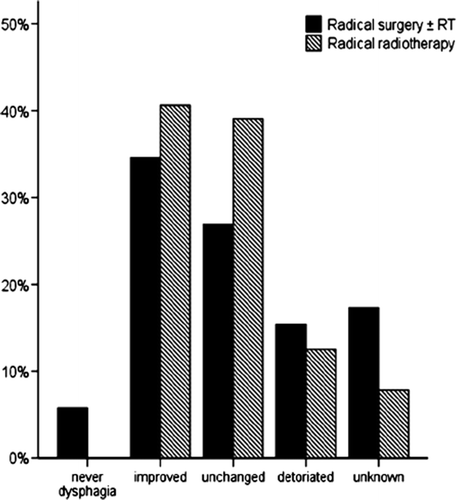
The level of dysphagia before treatment is described in . Patients selected for surgery had less dysphagia compared with patients selected for radical radiotherapy.
Information on dysphagia after treatment was recorded for 87% (161/184) of the patients. Some of them were evaluated shortly after treatment before leaving hospital while others had a later follow-up examination at the NRH. Of the 161 patients, 39 had no swallowing difficulties, 65 could eat solid food, 35 could eat soft food, while 22 could only swallow fluids. Changes in dysphagia from pre-treatment score to post-treatment score are illustrated for the two groups in .
Survival, tumor status and cause of death
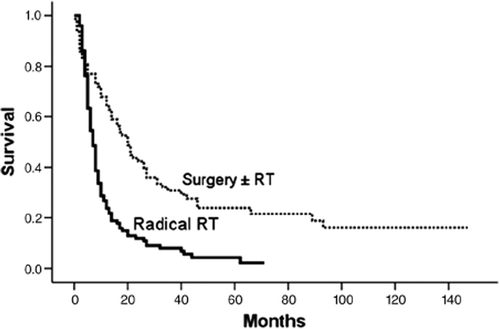
The 1-, 2- and 3-year survival for patients treated with radical surgery were 63%, 42% and 29%, while the corresponding figures for patients treated with radical radiotherapy were 24%, 12% and 8%. When excluding patients with lymph node or distant metastases on CT scans, the 1-, 2- and 3-year survival for patients treated with radical surgery were 67%, 44% and 32% while for patients treated with radical radiotherapy were 32%, 16% and 11%. The overall median crude survival, tumor status and causes of death are listed in .
Table III. Overall survival, tumor status at death or three years after start of treatment and causes of death
The survival of all patients treated with curative intent divided by treatment modality is presented in .
Figure 2. Overall survival for patients divided by treatment (n = 183). Overall survival for patients treated with surgery with or without preoperative radiotherapy (dotted line), a total of 17 patients were censored. Overall survival for patients treated with radical radiotherapy (RT) (continuous line), a total of four patients were censored.
Clinical factors associated with survival
Treatment given, performance status, weight loss, clinical stage and length of tumor were significantly associated with survival (p ≤ 0.05) in the univariate analyses (), while, age, sex and histology were not. In the Cox regression analysis of overall survival tumor length (< 5 cm favourable), gender (female favourable) and treatment (surgery ± radiotherapy favourable) were included as significant factors in the final model.
Table IV. Association between clinical factors and survival (Univariate analyses)
Discussion
In this 10-year cohort from a non-selected population of patients receiving curative treatment for oesophageal cancer, more than 50% were rejected for surgery, usually because of co-morbidity, high age, poor general condition or combinations thereof. Few patients eligible for surgery is not unique to our institution but has also been reported in other retrospective studies of unselected cohorts of patients [Citation13].
Our study demonstrates the poor prognosis of patients with cancer of the oesophagus, even when selected for curative treatment. Median survival was nine months and 3-year survival was 17% for all patients (n = 184). This is comparable to similar studies of curative treatment with median survival ranging from eight to 17 months [Citation14–16] and 3-year survival from 6 to 30% [Citation15,Citation17].
Patients treated with surgery ± radiotherapy had a significant longer median and 3-year survival than patients treated with radiotherapy alone which supports the strong position of surgery as the standard curative treatment for oesophageal cancer. However, survival in the operable patients was also limited with a median survival of less than two years and a treatment-related mortality of 9% (7/ 81) which is comparable with other studies [Citation13]. Clinical stage, treatment, performance status, weight loss and tumor length were significantly associated with overall survival in the univariate analyses as demonstrated in other studies [Citation5]. However, in the multiple Cox regression analysis, only tumor length and treatment were included in the final model in addition to gender. This might be explained by the use of radical radiotherapy in patients with reduced performance status, increased weight loss and more advanced disease. The female gender association cannot be further explained but has also been reported in other studies [Citation16].
The radical hyperfractionated radiotherapy regimen presented in the current paper is based on a randomized Scandinavian multi-centre study [Citation2]. Despite many negative prognostic factors (higher age, more often advanced disease, longer tumor, lower performance status, more weight loss and more symptoms than patients selected for surgery), patients treated with this regimen had results comparable with data from other studies () [Citation11,Citation18–25]. As expected, the survival was higher in patients treated with surgery. On the other hand, in the radiotherapy group, 11% of the patients with local disease were alive at three years. In a UK retrospective study of 32 consecutive patients treated with radical radiotherapy followed by brachytherapy, the 1-, 2- and 3-year survival of 34%, 16% and 16% found is comparable with our results [Citation18]. A higher survival was found in a Japanese study of 66 patients with local disease treated with high dose external radiotherapy followed by a brachytherapy; the 2- and 5-year survival were 37% and 18% respectively [Citation19].
Table V. Comparison of results between radical radiotherapy at the Norwegian Radium Hospital and results from other radical radiotherapy trials
The hyperfractionated accelerated regimen that we used was well tolerated. Only 20% of the patients experienced complications compared to more than 50% of the patients treated with surgery ± radiotherapy. The complications reported were mostly acute infections treated with antibiotics or late stenoses palliated with dilatations. Only two patients experienced serious events such as fistulas.
The possible advantages of using accelerated hyperfractionated regimens have been explored and supported by Japanese and Chinese authors. A randomized study of hyperfractionated accelerated radiotherapy compared to conventional fractionated radiotherapy demonstrated a trend towards longer survival without increased toxicity for the hyperfractionated regimen [Citation11]. In another prospective randomized study comparing late course accelerated hyperfractionated radiotherapy (LCAF) to conventional radiotherapy (CF), significantly longer median and 5-year survival was found in the LCAF group [Citation20]. Furthermore, in a Chinese prospective study of 201 patients with local disease receiving LCAF, the 1-, 3- and 5-year overall survival rates were 73%, 34% and 26%, respectively which is comparable to the results in the chemoradiation-arm in the study by Herskovic and co-workers [Citation21,Citation23]. On the other hand, in a study of 101 patients randomized to continuous accelerated hyperfractionated radiotherapy (CAHF) compared to late-course accelerated radiotherapy (LCAF), there were no significant differences in the 1-, 2,- and 3- year survival. In addition CAHF resulted in more severe acute osophagitis and might be less well tolerated than LCAF [Citation22]. In a review by Ask and co-workers accelerated hyperfractionated radiotherapy was supported as potentially being superior to conventional radiotherapy [Citation4].
To improve the discouraging results on survival, the possible advantage of adding chemotherapy to the treatment regimens has been explored in patients fit for such treatment. Review articles conclude that there is a trend toward superior outcome for patients who receive preoperative chemoradiotherapy compared to surgery alone [Citation5]. Standard treatment for inoperable patients with local disease has been accepted to be concurrent chemotherapy and radiotherapy (CiFu and 50 Gy) in many centres based on the well known studies by Herskovic and co workers and Minsky and co-workers [Citation23, Citation24]. However, the clinical reality is that many patients are not candidates for chemotherapy for the same reasons that they were found medically inoperable. There is no consensus on how to treat this large group of patients. Thus, it is very important to evaluate treatment results of non-surgical patients with a curative potential who cannot tolerate chemotherapy.
Conclusion
There is a strong need to establish adequate treatment-options for patients with curable oesophageal cancer and contraindications for surgery and/or chemoradiotherapy. The current hyperfractionated radiotherapy provides symptom relief without extensive toxicity and with a possibility for cure. The literature (although mostly from eastern countries) strongly supports the curative potential of high dose accelerated hyperfractionated radiotherapy even though the optimal radiotherapy regimen still needs to be explored.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- The Cancer Registry of Norway. Cancer in Norway 2001.
- Nygaard K, Hagen S, Hansen HS, Hatlevoll R, Hultborn R, Jakobsen A, . Pre-operative radiotherapy prolongs survival in operable esophageal carcinoma: A randomized, multicenter study of pre-operative radiotherapy and chemotherapy. The second Scandinavian trial in esophageal cancer. World J Surg 1992;16:1104–10.
- Mala T, Løtveit T. Surgical treatment of oesophageal cancer. Tidsskr Nor Laegeforen 2001;121:2815–7.
- Ask A, Albertsson M, Järhult J, Cavallin-Ståhl E. A systematic overview of radiation therapy effects in oesophageal cancer. Acta Oncol 2003;42:462–75. Review.
- Koshy M, Esiashvilli N, Landry JC, Thomas CR, Matthews RH. Multiple management modalities in esophageal cancer: Epidemiology, presentation and progression, work-up, and surgical approaches. Oncologist 2004;9:137–46.
- Earlam R, Cunha-Melo JR. Oesophageal squamous cell carcinoma: I. A critical review of surgery. Br J Surg 1980;67:381–90.
- Sykes AJ, Burt PA, Slevin NJ, Stout R, Marrs JE. Radical radiotherapy for carcinoma of the oesophagus: An effective alternative to surgery. Radiother Oncol 1998;48:15–21.
- Ekman S, Dreilich M, Lennartsson J, Wallner B, Brattström D, Sundbom M, . Esophageal cancer: Current and emerging therapy modalities. Expert Rev Anticancer Ther 2008;9:1433–48.
- Hatlevoll R, Hagen S, Hansen HS, Hultborn R, Jakobsen A, Mäntylä M, . Bleomycin/cis-platin as neoadjuvant chemotherapy before radical radiotherapy in localized, inoperable carcinoma of the esophagus. A prospective randomized multicentre study: The second Scandinavian trial in esophageal cancer. Radiother Oncol 1992;24:114–6.
- Wobbes T, Baron B, Paillot B, Jacob JH, Haegele P, Gignoux M, . Prospective randomised study of split-course radiotherapy versus cisplatin plus split-course radiotherapy in inoperable squamous cell carcinoma of the oesophagus. Eur J Cancer 2001;37:470–7.
- Nishimura Y, Ono K, Tsutsui K, Oya N, Okajima K, Hiraoka M, . Esophageal cancer treated with radiotherapy: Impact of total treatment time and fractionation. Int J Radiat Oncol Biol Phys 1994;30:1099–105.
- Statistics Norway. www.statbank.ssb.no.
- Stockeld D, Backman L, Fagerberg J, Granström L. Esophageal cancer in Stockholm county 1978–1995. Acta Oncol 2007;46:1075–84.
- Leslie MD, Dische S, Saunders MI, Grosch E, Fermont D, Ashford RF, . The role of radiotherapy in carcinoma of the thoracic oesophagus: An audit of the Mount Vernon experience 1980–1989. Clin Oncol (R Coll Radiol) 1992;4:114–8.
- Urba SG, Orringer MB, Turrisi A, Iannettoni M, Forastiere A, Strawderman M. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol 2001;19:305–13.
- Neuhof D, Neumayer F, Einbeck W, Haschemian K, Mai SK, Hochhaus A, . Retrospective evaluation of combined modality treatment and prognostic factors in patients with esophageal cancer. Acta Oncol 2005;44:168–73.
- Walsh TN, Noonan N, Hollywood DA, Kelly A, Keeling N, Hennessy TPJ. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med 1996;335:462–7.
- Churn M, Jones B, Myint AS. Radical radiotherapy incorporating a brachytherapy boost for the treatment of carcinoma of the thoracic oesophagus: Results from a cohort of patients and review of the literature. Clin Oncol (R Coll Radiol) 2002;14:117–22.
- Hishikawa Y, Kurisu K, Taniguchi M, Kamikonya N, Miura T. High-dose-rate intraluminal brachytherapy for esophageal cancer: 10 years experience in Hyogo College of Medicine. Radiother Oncol 1991;21:107–14. Review.
- Shi XH, Yao W, Liu T. Late course accelerated fractionation in radiotherapy of esophageal carcinoma. Radiother Oncol 1999;51:21–6.
- Zhao KL, Shi XH, Jiang GL, Wang Y. Late-course accelerated hyperfractionated radiotherapy for localized esophageal carcinoma. Int J Radiat Oncol Biol Phys 2004;60:123–9.
- Wang Y, Shi XH, He SQ, Yao WQ, Wang Y, Guo XM, . Comparison between continuous accelerated hyperfractionated and late-course accelerated hyperfractionated radiotherapy for esophageal carcinoma. Int J Radiat Oncol Biol Phys 2002;54:131–6.
- Herskovic A, Martz K, al-Sarraf M, Leichman L, Brindle J, Vaitkevicius V, . Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med 1992;326:1593–8.
- Minsky BD, Pajak TF, Ginsberg RJ, Pisansky TM, Martenson J, Ritsuko K, . INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: High-dose versus standard-dose radiation therapy. J Clin Oncol 2002;20:1151–3.
- Al-Sarraf M, Martz K, Herskovic A, Leichman L, Brindle JS, Vaitkevicius VK, . Progress report of combined chemoradiotherapy versus radiotherapy alone in patients with esophageal cancer: An intergroup study. J Clin Oncol 1997;15:277–84.