Abstract
Background. Cyclin E, a key regulator in the cell cycle, is often over-expressed in malignant disease. It can present as full length (FL) and low-molecular-weight (LMW) isoforms. The purpose of this study was to characterize the expression pattern of cyclin E in colon cancer, both in tumor and in macroscopically normal adjacent mucosa. A secondary aim was to study the possible correlation to clinical factors and patient outcome. Material and method. Tumor and mucosa tissue from 114 patients with radically operated, non-metastatic colon tumors were analyzed. The cyclin E expression was measured by Western Blot in the tumor and adjacent mucosa using the antibody targeting C-terminal. The cyclin E expression was correlated to both pathology factors as differentiation grade and to the patient outcome. Results. Cyclin E was detected in both tumor and adjacent mucosa and in both FL and LMW-forms. FL was present in 29 (25.4%) tumors and only in three (2.6%) mucosa samples, the corresponding figures for the LMW-isoforms were 80 (70.2%) and 67 (58.8%). There was no correlation between the cyclin E expression and gender, age, tumor location or tumor pathology. Patients with a high expression of LMW isoforms (p < 0.03) or a high total expression (FL+LMW) (p < 0.006) had higher risks of recurrence and thus a worse survival. Conclusion. Cyclin E is expressed in FL- and LMW-forms in both colon tumors and the macroscopically normal adjacent mucosa. A high expression of cyclin E in tumor was associated with an increased risk of tumor recurrence and a worse outcome. It could be a possible prognostic marker in non-metastatic colon cancer.
Colon cancer is one of the most common cancer forms in Western Europe. The main curative treatment is surgical removal of the tumor. Important prognostic factors include the presence of lymph node involvement or distant metastases [Citation1]. In the early stages of disease, without evidence of spread, the patient should be cured and the prognosis good. Still, up to 15–20% suffers a tumor recurrence. Finding markers to identify the high risk individuals would be of both prognostic and therapeutic interest. Recently, cyclin E and its low molecular weight (LMW) forms has been reported to be the one of the most prominent prognostic factors in breast cancer [Citation2]. It has been shown that small and node-negative breast tumors with high expression of LMW-isoforms of cyclin E carry a poor prognosis despite the tumor grade. It has also been shown that estrogen-receptor positive breast tumors with overexpression of LMW-isoforms has a worse response to antiestrogen treatment [Citation3].
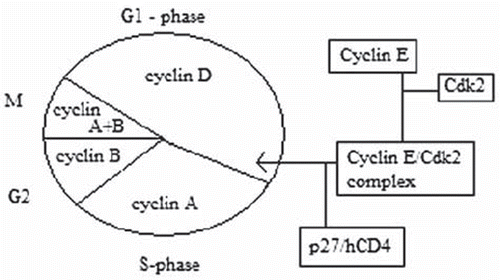
Cyclin E is an activator of cyclin dependent kinase and regulates the transition from the G1 to the S phase in the mammalian cell division cycle (). Thus, it is critical for the initiation of DNA replication and other S-phase functions. The E-type cyclin functions together with the D-types by integrating the positive growth stimuli of mitogenic signals to promote cellular proliferation. High levels of cyclin E can accelerate the transition through the G1 phase and decrease cell size [Citation4]. In tumor cells, cyclin E can be deregulated by a number of mechanisms such as: gene amplification [Citation5], down-regulation of the associated p27-system [Citation6] and downregulation of its specific F-box protein Fbw7 (hCDC4) which tags phosphorylated cyclin E for proteosomal degradation [Citation7]. Mutations in hCDC4 regulation genes have been found in breast, ovarian, endometrial and colorectal cancers and were also associated with elevated levels of cyclin E protein [Citation8].
The cyclin E protein can be expressed in a full length (FL-50 kD) form and also as a LMWisoform. The isoforms are created by the proteolytic cleavage of the FL protein and ranges in size from 45 to 33 kD. The LMW-isoforms can be biochemically hyperactive, compared to the FL protein, in phosphorylating substrates and in inducing progression in the cell cycle [Citation9]. These results suggest that an over-expression of the LMW forms of cyclin E stimulates the cells to progress through the cell cycle much more efficiently than the full length cyclin E [Citation10]. The cyclin E and its isoforms have been characterized in ovarian cancer [Citation11], gastric cancer [Citation12] and also colon cancer [Citation13]. There are reports showing bio-molecular abnormalities in the mucosa adjacent to a tumor. Therefore, an analysis of the expression of cyclin E in the macroscopically normal mucosa was included.
The aim of the study was to explore the expression of cyclin E and its LMW-isoforms in colon tumors and adjacent mucosa. A secondary aim was to investigate the possible correlation of the cyclin E expression to the prognosis and outcome in patients with early colon cancer.
Material and methods
Patients and material
For the study 114 patients with surgically treated for non-metastasized colon tumors was selected. They were randomly selected out of the 288 patients radically operated for stage I or II colon cancer during the period 2003–2007. The study was approved by the local Ethics committee and all the patients had given their written informed consent. The preoperative staging was performed with chest x-ray and liver exam (CT or ultra-sound). All the patients were treated along the same guidelines including the regular postoperative clinical and radiological surveillance. Basic clinical parameters as gender, age, diagnosis, cancer location and performed surgery as well as pathology data such as lymph nodes and differentiation grade was retrieved from journals and charts. Follow-up data was obtained on cancer status, survival and possible recurrences including their location was retrieved from a continuously updated outcome registry. Biological samples had been prospectively gathered and were available for all patients. The cyclin E expression was assessed with Western Blot in both tumor and in the macroscopically normal adjacent mucosa, 5–10 cm from the tumor, for both FL and LMWisoforms.
Western blot assays
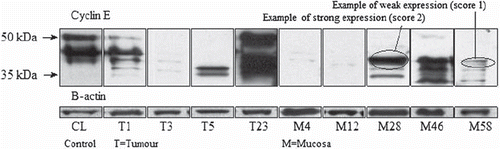
Levels of FL cyclin E and LMW-isoforms were evaluated by Western blot analysis of lysates prepared from the frozen tumor tissues and adjacent mucosa. Cell pellets were homogenized by sonication in one volume of sonication buffer (50 mM Tris-HCl ph 7.4, 0.25 M NaCl, 1 mM dithiothreitol) at 4°C with a following centrifugation at 15 000 g for 20 min at 4°C. Then 50 μg of proteins from each sample were electrophoresed in a 10% SDS-polyacrylamide gel (SDS-PAGE) and transferred to Nitrocellulose membrane (Biorad). The blots were blocked overnight at 4°C in Blotto (5% non-fat dry milk in 20 mM Tris, 137 mM NaCl, and 0.25% Tween). After six 10 min washes in TBS-T (20 mM Tris, 137 mM NaCl, and 0.05% Tween), the blots were incubated in primary antibodies for one hour. The primary antibodies used were: monoclonal antibody HE12 to cyclin E (1:500; Santa Cruz Biotechnology, CA), actin monoclonal antibody (1:500; Santa Cruz Biotechnology, CA. All antibody dilutions were made in Blotto. After primary antibody incubation, the blots were washed and incubated with the appropriate goat anti-mouse horseradish peroxidase conjugate (1:5 000) in Blotto for one hour, then washed and developed with the ECL chemiluminesence reagent (Amersham) as directed by the manufacturer. Equivalent amount of protein from the control cell line HT29 was included on the gel as by internal laboratory standard. Actin staining was also used to normalize for protein loading. For each gel, the equal amount of cell line HT29 extracts were included to serve as positive control. The positive control was used to allow accurate comparison of cyclin E and its LMW-isoforms across all the different gels. Multiple different image exposures were taken for each gel. To be able to compare these different exposures, the gels were selected in such a way that density expression for the positive controls on each gel was as close to each other as possible.
Scoring and analysis
The level of Cyclin expression for each variant was classified on an ordinal scale from 0 (none) through 1 (weak) to 2 (strong). The scoring was done optically in a blinded manner by two persons without knowledge of clinical or pathology data. In case of a discrepancy, it was resolved by an independent third part. Examples of the scoring levels for the expression are shown in . The total cyclin expression was computed by adding the FL and LMW figures, acquiring a total score ranging from zero to four. The data, also grouped by the total cyclin E expression, was then compared and analyzed against both demography and pathology parameters. The outcome was assessed by cancer specific survival (CSS) related to the FL and LMW forms and the total cyclin E expression. Also, in order to validate the findings of the LMW isoforms and show that it was not a matter of tissue degradation, an additional 10 samples of biological material was also tested after an intentional mistreating in the preparation.
Statistical method
The JMP 4 /SAS software was used for statistical analysis (SAS institute). The basic patient demographic data was set by distribution statistics with ANOVA or contingency tables for the non-parametric data with χ2 (Pearson) analysis. The Kaplan-Meier regression method with Log-rank test was used to assess survival. Due to the low number of events in the material a multivariate analysis was not included. The confidence level was set at 95%.
Results
Patients and demography
The patient demography data are presented in . The median age of the patients was 71 years. There was an even gender distribution. The preoperative staging was completed in 99% of elective patients. Tumor location in right colon was slightly more frequent in this group and right hemicolectomy was the most common surgical procedure. All had radical removal of the tumor. A total of seven patients received adjuvant chemotherapy but none in the high expression group. One patient was treated by emergency surgery but did not differ in any other aspects. For this patient the staging was completed postoperative and then with normal results. The analyzed 114 patients, as a group, did not significantly differ from the entire stage I and II cohort during this period. The median follow-up time was 42 months.
Table I. Patient demography and pathology data.
Cyclin E expression in the adjacent mucosa
The expression of cyclin E was as presented in . It was mainly the LMW-isoforms of cyclin E that was expressed in the mucosa samples. Sixty seven patients (58.8%) had an expression of cyclin E in the colon mucosa. The association between the prevalence of cyclin E in tumor and mucosa is shown in . In 16 patients, the expression was absent in tumor and positive in mucosa. The opposite, with a positive expression in tumor and negative in mucosa, was more common and seen in 31 patients.
Table II. The expression of cyclin E in colon and the association between FL and LMW isoform expression (n(%)) in adjacent mucosa and tumour in stage I/II colon cancer (n=114).
Table III. The association between total expression in tumour and total expression in adjacent mucosa of Cyclin E in both full length (FL) form and in the LMW-isoform (n(%)) in stage I/II colon cancer (n=114).
Cyclin E expression in the tumor
Both FL and LMW-isoforms were present in the colon cancer tissue as presented in . The FL form was expressed weakly in 14 patients (12.3%) and strongly expressed in 15 patients (13.2%). The LMW forms were expressed weakly in 56 (49.1%) and strongly in 24 (21.1%) patients. Thirty patients (26.3%) had no cyclin E expression whilst it was very heavily expressed in nine patients (7.9%). The expression patterns had a significant correlation (Pearson, p < 0.0014), with a higher expression of the FL form being more likely to also express the LMW-isoforms. The expression patterns of cyclin E showed a large individual variation and it can be expressed in a wide range of possibilities.
Scoring and test of tissue degradation
Examples of both the cyclin expressions as well as examples of grading of the expression strength are shown in . The inter-observer variability with the scoring of cyclin E expression was very small. Discrepancies occurred for <10% of the samples and were then resolved by a third party umpire. The 10 samples tested for tissue degradation were unanimously assessed. The pattern and positions of bands recognized with anti-cyclin E antibody significantly varied from the pattern of cyclin E from the fresh frozen tissue and were easily distinguishable in all 10 cases. The choice of the method of tissue disruption or protein isolation did not influence the WB image of cyclin E.
Pathology and outcome
The pathology data, grouped by the total cyclin E expression level, is described in . There was no difference in lymph node assessment or in differentiation grade by the cyclin E expression group. Nor was there any difference in demography or in local T-stage. There was a significantly higher risk of recurrence for the patients with a high expression of both the FL and the LMW forms in the tumor (p < 0.01). This risk was also associated with an increased risk of multiple metastasis locations (p < 0.01). A significantly worse survival was also observed in that group (, Log rank, p < 0.0061). A high expression of the LMW-isoform itself was associated to a worse survival (p < 0.03) whilst the FL-form was not (p < 0.07). There was no statistical association between the expression of cyclin E in the mucosa and the patient outcome.
Figure 3. Cancerspecific survival in stage III colon cancer, grouped by total cyclin E expression in the tumor (1-low expression. 4-high total expression).
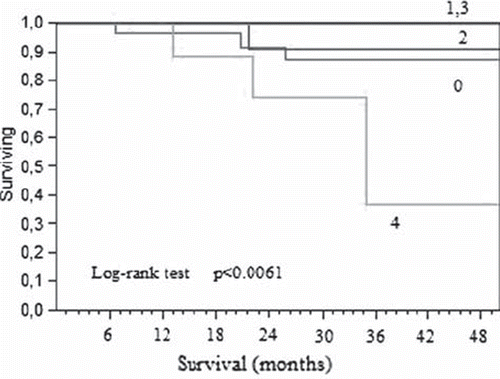
Table IV. Demography and pathology data by the total Cyclin E expression in the tumour
Discussion
A radically operated colon cancer, without evidence of metastasis presence, should have a good survival prognosis. Still, there is a group of high risk patients with poor outcome despite optimal surgery and staging routines. Even in stage II disease there is a 10–20% risk of dying from a cancer recurrence. Finding markers that can aid in the identification of these patients could possibly improve the treatment and survival in that group. Cyclin E is interesting as it participates in the regulation of the cell cycle by affecting the transition from the G1 to the S phase. Animal studies has shown that an over-expression of cyclin E can affect and disturb the cell-cycle control in benign adenomas, and thereafter also participate in tumor progression [Citation14]. Increased levels of cyclin E are suggested to promote the transition from adenoma to adenocarcinoma, a key step in the colorectal carcinogenesis [Citation15]. Accordingly, an aberrant expression of cyclin E has been found in a significant fraction of human colon carcinomas [Citation16]. These findings concur with our results that cyclin E is expressed in the colon tumor tissue.
Colorectal cancer cells expresses not only the FL form but also the LMW isoforms of cyclin E, both in vitro and in vivo [Citation13]. The LMW isoform was thought to be unique to tumor cell lines and tissues. However, we found cyclin E expression not only in colon cancer tissue but also in the surrounding mucosa. Interestingly, other studies have shown that the colorectal mucosa in patients with colorectal cancer is characterized by alterations in the DNA, RNA, and protein levels [Citation17]. One example is by Chen et al., who reported that several inflammation markers were altered in mucosa of patients with colorectal cancer as compared with controls [Citation18]. Thus, it is plausible that the colorectal mucosa in patients with colon cancer differs from that of healthy individuals on several levels. Even though we were unable to correlate the Cyclin E expression in the mucosa to the pathology, the mere finding is still of interest and does also challenge the enzymes tumor specificity.
There are currently two suggested mechanisms for the formation of cyclin E LMW-isoforms. First, as shown by Porter et al., the tumors produce extracellular elastase, which can cleave FL cyclin E into LMW-isoforms [Citation9]. The secreted elastase could come into contact with the intracellular cyclin E and induce the cleavage in lysed cells and thus induce the isoforms. This could explain the appearance of LMWisoforms of cyclin E in adjacent mucosa closely surrounding the tumuor. Second, as shown by Libertini et al., the calpain 2 has been discussed as a possible protease participating in cyclin E cleavage [Citation19]. However, it is questionable if it can explain the cyclin E isoforms present at 10 cm distance from the tumor. Possibly, it could be a sign of inflammation or they could serve other functions in the mucosa. One hypothetical function could be in enhancing angiogenesis [Citation20,Citation21] which then also could increase the metastasis potential [Citation12].
The study shows that cyclin E is expressed in colon cancer, in both full length and LMW isoforms and that it can be detected in the tumor tissue as well as in the adjacent mucosa. Also show is a possible association between the cyclin E expression and the clinical outcome. In contrast to Lim et al., who reported no association [Citation22], we believe that cyclin E is of importance also in colon cancer. It would then concur with previous findings where cyclin E overexpression has been reported to be an indicator of poor outcome in several tumors like breast cancer, gastric cancer and ovarian cancer. The aggressive clinical tumor characteristics associated to high Cyclin E expression does also concur with experimental studies and clinical data from other types of neoplasia. The outcome results and their interpretation are limited in the low number of follow-up events. However, we find it notable that half of them occur in the small high expression group and that most of them are multi-location recurrences making the prognosis even worse. The studied early cancer stages normally have a good prognosis which makes these findings even more interesting.
Another possible weakness is the difficulty in grading the cyclin E expression. Whilst a densitometry analysis adds figures in that aspect it also brings the risk of arbitrarily set cut-off levels. The optically based three point scale was in our opinion functional. Similar scoring system has also been used previously by other authors [Citation2]. The homogeneity of the patient and pathology data does in our opinion also add strength to the study. The material is of a special nature since we have both tumor and mucosa material and all linked to a solid clinical record and follow-up. Important is also the quality of the performed staging and foremost the lymph node assessment. The results show that cyclin E could be a possible prognostic factor in early colon cancer, but the findings should be validated in larger series as well as in other cancer stages. It would also be of interest to study if and how cyclin E is expressed by immune-active cells, like macrophages, and that the findings thus could be linked to characteristics of associated pathology.
Conclusion
Cyclin E is expressed in FL- and LMW-forms in both colon tumors and the macroscopically normal adjacent mucosa. A high expression of cyclin E in tumor was associated with an increased risk of tumor recurrence and a worse outcome. It could be a possible prognostic marker in non-metastatic colon cancer.
Acknowledgement
We would like to thank the Swedish Cancer Society, the Göteborg University and the Bror Björnsson Foundation for their financially support of our work. The study was in part performed at Proteomics Core Facility, The Sahlgrenska Academy, University of Gothenburg.
Declaration of interest: The authors report no conflicts of interest. The author alone are responsible for the content and writing of the paper.
References
- Crawford NP, Colliver DW, Galandiuk S. Tumor markers and colorectal cancer: Utility in management. J Surg Oncol 2003;84:239–48.
- Keyomarsi K, Tucker SL, Buchholz TA, Callister M, Ding Y, Hortobagyi GN, . Cyclin E and survival in patients with breast cancer. N Engl J Med 2002;347:1566–75.
- Akli S, Zheng PJ, Multani AS, Wingate HF, Pathak S, Zhang N, . Tumor-specific low molecular weight forms of cyclin E induce genomic instability and resistance to p21, p27, and antiestrogens in breast cancer. Cancer Res 2004;64:3198–208.
- Resnitzky D, Gossen M, Bujard H, Reed SI. Acceleration of the G1/S phase transition by expression of cyclins D1 and E with an inducible system. Mol Cell Biol 1994;14:1669–79.
- Cassia R, Moreno-Bueno G, Rodriguez-Perales S, Hardisson D, Cigudosa JC, Palacios J. Cyclin E gene (CCNE) amplification and hCDC4 mutations in endometrial carcinoma. J Pathol 2003;201:589–95.
- Bloom J, Pagano M. Deregulated degradation of the cdk inhibitor p27 and malignant transformation. Semin Cancer Biol 2003;13:41–7.
- Strohmaier H, Spruck CH, Kaiser P, Won KA, Sangfelt O, Reed SI. Human F-box protein hCdc4 targets cyclin E for proteolysis and is mutated in a breast cancer cell line. Nature 2001;413(6853):316–22.
- Rajagopalan H, Jallepalli PV, Rago C, Velculescu VE, Kinzler KW, Vogelstein B, . Inactivation of hCDC4 can cause chromosomal instability. Nature 2004;428(6978):77–81.
- Porter DC, Zhang N, Danes C, McGahren MJ, Harwell RM, Faruki S, . Tumor-specific proteolytic processing of cyclin E generates hyperactive lower-molecular-weight forms. Mol Cell Biol 2001;21:6254–69.
- Wingate H, Bedrosian I, Akli S, Keyomarsi K. The low molecular weight (LMW) isoforms of cyclin E deregulate the cell cycle of mammary epithelial cells. Cell Cycle 2003;2:461–6.
- Bedrosian I, Lu KH, Verschraegen C, Keyomarsi K. Cyclin E deregulation alters the biologic properties of ovarian cancer cells. Oncogene 2004;23:2648–57.
- Milne AN, Carvalho R, Jansen M, Kranenbarg EK, van de Velde CJ, Morsink FM, . Cyclin E low molecular weight isoforms occur commonly in early-onset gastric cancer and independently predict survival. J Clin Pathol 2008;61:311–6.
- Corin I, Di Giacomo MC, Lastella P, Bagnulo R, Guanti G, Simone C. Tumor-specific hyperactive low-molecular-weight cyclin E isoforms detection and characterization in non-metastatic colorectal tumors. Cancer Biol Ther 2006;5:198–203.
- Hur K, Kim JR, Yoon BI, Lee JK, Choi JH, Oh GT, . Overexpression of cyclin D1 and cyclin E in 1,2-dimethylhydrazine dihydrochloride-induced rat colon carcinogenesis. J Vet Sci 2000;1:121–6.
- Yasui W, Kuniyasu H, Yokozaki H, Semba S, Shimamoto F, Tahara E. Expression of cyclin E in colorectal adenomas and adenocarcinomas: Correlation with expression of Ki-67 antigen and p53 protein. Virchows Arch 1996;429:13–9.
- Sutter T, Doi S, Carnevale KA, Arber N, Weinstein IB. Expression of cyclins D1 and E in human colon adenocarcinomas. J Med 1997;28:285–309.
- Feng M, Pei F, Yang G, Mao Z, Wang S. Expression of p53 and p21 protein in transitional mucosa adjacent to rectal carcinoma and its clinical implication. J Tongji Med Univ 2000;20:220–1.
- Chen LC, Hao CY, Chiu YS, Wong P, Melnick JS, Brotman M, . Alteration of gene expression in normal-appearing colon mucosa of APC(min) mice and human cancer patients. Cancer Res 2004;64:3694–700.
- Libertini SJ, Robinson BS, Dhillon NK, Glick D, George M, Dandekar S, . Cyclin E both regulates and is regulated by calpain 2, a protease associated with metastatic breast cancer phenotype. Cancer Res 2005;65:10700–8.
- Bales E, Mills L, Milam N, McGahren-Murray M, Bandyopadhyay D, Chen D, . The low molecular weight cyclin E isoforms augment angiogenesis and metastasis of human melanoma cells in vivo. Cancer Res 2005;65:692–7.
- Akli S, Van Pelt CS, Bui T, Multani AS, Chang S, Johnson D, . Overexpression of the low molecular weight cyclin E in transgenic mice induces metastatic mammary carcinomas through the disruption of the ARF-p53 pathway. Cancer Res 2007;67:7212–22.
- Lim YJ, Kim YH, Ahn GH, Chun HK, Jang WY, Lee JH, . Cyclin E, p27 and mutant p53 do not predict the prognosis in AJCC stage II colorectal carcinomas. Korean J Gastroenterol 2004;44:314–20.