Abstract
Introduction. High levels of certain matrix metalloproteinases (MMPs) have been detected in various human cancers. The purpose of this study was to analyze the expression of MMP-7 in salivary gland cancer (SGC) by immunohistochemistry and to associate the results with the clinical data and the 10-year survival of the SGC patients. Material and methods. Immunohistochemistry for MMP-7 was performed in a series of 107 paraffin-embedded sections of SGC. The samples represent the entire SGC population in Finland from 1991–1996. Mortality follow-up ended December 31, 2006. Results. The study population of 107 patients consisted of 47 male and 60 female subjects, ranging in age at the time of diagnosis between 23 and 90 years. The minimum follow-up time was 10.6 years and the maximum 15.9 years. By age-adjusted analysis lower staining intensity was associated with worse overall survival of patients with acinic cell carcinoma (p = 0.047, HR 6.5, 95% Cl 1.0–41.7) and in mucoepidermoid carcinoma (p = 0.010, HR 9.3, 95% CI 1.7–50.0). Low staining intensity was also associated with worse disease-specific survival of patients with acinic cell carcinoma (0–1 vs. 2–3; p = 0.047, HR 13.7, 1.0–200.0). VCI Ki-67 was an important prognostic factor for survival of the entire data set (p < 0.0001, HR 4.7, 95% Cl 2.3–9.8). Conclusions. MMP-7 is associated with the prognosis of patients with acinic cell and mucoepidermoid carcinoma.
Salivary gland carcinomas (SGCs) are uncommon neoplasms and account for only 3–6% of all head and neck malignancies [Citation1]. The occurrence varies strongly in different parts of the world and the incidence ranges from 0.4 to 2.6 per 100 000 population [Citation2]. In Finland, the age-adjusted incidence in was 0.8 for males and 0.7 for females per 100 000 person-years in 2006 [Citation3]. Salivary gland neoplasms may occur in any of the salivary glands. Approximately 70–80% arises in the parotid gland, which is also the most common location of SGC among patients in Finland (64%) [Citation4]. The relative 5-year survival rate for acinic cell cancer is 96%, mucoepidermoid cancer 79% and adenoid cystic cancer 74% [Citation4].
The response to treatment varies by not only histological type but also within the particular histological types, an observation that is obviously related to the biological heterogeneity of these tumors. This heterogeneity complicates the use of prognostic tools for determination of the aggressiveness of these neoplasms. Apart from disease extent, there are no well-known factors that determine the prognosis of patients with SGC. The treatment of the patients needs to be tailored individually based on the histological diagnosis and on classification of the tumor in a way that reflects the propensity of late recurrence [Citation5].
The matrix metalloproteinases (MMPs) are a family of zinc-dependent proteolytic enzymes capable of degrading the extracellular matrix (ECM). Based on their structure and substrate specificity, the MMPs are divided into the following groups: collagenases, gelatinases, stromelysin, matrilysins, membrane-type MMPs (MT-MMPs) and other MMPs. Common to all MMPs is their ability able to break down all ECM components, including collagens, elastin, proteoglycans, laminin and fibronectin. MMPs play a key role in the physiological degradation of ECM in angiogenesis, tissue repair and tissue morphogenesis. They also regulate cell growth and inflammation by cleaving non-matrix proteins like growth factors, cytokines and chemokines and their respective receptors [Citation6]. High levels of MMPs have been associated with the invasive properties of cancer and their role as a prognostic factor has been studied in different cancer types [Citation7,Citation8].
Matrilysin (MMP-7) is the smallest molecule of the MMPs, as it lacks the COOH-terminal hemopexin-like domain. This domain probably defines substrate specificity [Citation9] and the absence of this domain is in conformity with the observation that matrilysin exhibits high activities against a wide range of substrates, including components of the basement membrane, e.g., collagen, laminin and entactin [Citation9].
Matrilysin is mainly expressed by non-injured, non-inflamed exocrine and mucosal epithelium. It is produced by skin and salivary glands, ciliated cells of the respiratory tract and by the ductal or glandular epithelium of the pancreas, liver, breast, intestine and urogenital tissues. Malignant epithelial cells in tumors of the gastrointestinal tract, lung, prostate and breast also express matrilysin [Citation8–10]. MMP-7 activates defensins, i.e., antibacterial peptides necessary for the defence of the intestinal mucosa [Citation11]. MMP-7 processes extra cellular matrix components as well as cell surface molecules, e.g. pro-α-defensin, Fas-ligand, pro-tumor necrosis factor (TNF)-α and E-cadherin [Citation12]. MMP-7 inhibits tumor angiogenesis in vitro and in vivo by generating angiostatin [Citation13].
MMP-7 is widely expressed in healthy cells and also in tumor cells, especially at the invading front of tumors, while other MMPs, in addition, present in the surrounding stromal cells and the inflammatory cells involved in cancer progression [Citation10].
The purpose of the present study was to analyze the immunohistochemical expression of MMP-7 and to examine the association between MMP-7 expression and 10-year overall survival of a nationwide population of patients with SGC in Finland.
Patients, materials and methods
The data of was derived from the SGC cases diagnosed in Finland and reported to the Finnish Cancer Registry (FCR) during 1991–1996 [Citation4]. This data represent the entire population of Finland. The patient records were scrutinized and the 5- and 10-year survival data was received from the Statistic Finland.
Cancer tissue specimens of all available adenoid cystic carcinomas (AdCC = 47; 44%), mucoepidermoid carcinomas (MEC = 23; 22%), acinic cell carcinomas (AcCC = 24; 22%) and salivary duct carcinomas (SDC = 13; 12%) were studied. Only 107 samples of the original 164 (65%) histological blocks were available, since the some of the paraffin blocks had been used up in previous studies.
Immunohistochemistry
All immunohistochemical stainings were performed on formalin-fixed and paraffin-embedded tissue samples. Immunohistochemical stainings were performed using a BenchMark XT (Ventana Medical Systems, Inc., Tucson, AZ, USA) immunostaining machine and UltraView Universal DAB Detection kit (Ventana Medical Systems, Inc., Tucson, AZ, USA). A mouse monoclonal antibody Anti-MMP-7, Mouse mAb ID2, Thermo Scientific dilution, 1:10 was used as a primary antibody. The pH at epitope retrieval of the antigens was 8.4. The intensity of cytoplasmic staining (0 = no staining, 1 = weak, 2 = moderate and 3 = strong cytoplasmic staining) and the percentage of positive cells in the area of the strongest staining were recorded at a 250–400-fold magnification.
Multiplicates of the staining intensity and the percentage of the positive cells were used as an index and constituted the primary result variable. The method is semi-quantitative. To improve standardization, the stained sections of healthy salivary gland samples served as a control. The concordance of the staining pattern was assessed by inspection through a microscope (Leitz, Dialux 22, Viereich Germany) by consultants H.L. and P.K., who were blinded to the patients’ clinical status. Several representative fields were examined for evaluation. Discordant cases were discussed, and after consensus the result was recorded. In previous studies of MMP expression in salivary gland cancer the intensity of cell staining has been ignored, but in this study the use of a fully automatic immunostaining device allowed this feature to be analyzed, which improved the reliability of our scoring.
Statistical methods
The follow-up time was calculated from the date of diagnosis to the date of death or the end of data collection (December 31, 2006). Univariate, age-adjusted and multivariate Cox proportional hazards models were used to estimate the associations between MMP-7, age, gender, tumor location, histological type and TNM stage with the patients’ disease-specific and overall survival. MMP-1, -9, -13 and volume-corrected Ki-67 positive cells per square mm of tumor tissue (VCI Ki-67) were included in the statistical analysis, since we have studied these variable previously for the same material [Citation14,Citation15]. The percent of staining cells and the MMP-7 index were divided into three categories by quartiles so that the lowest quartile formed category 1, the 2nd and 3rd quartile combined formed category 2 and the highest quartile formed category 3. The results were expressed as hazard ratios with 95% confidence intervals (95% CI). The statistically significant explanatory variables in the age-adjusted models were then included in the multivariate analysis. In all analyses, a two-sided p-value of less than 0.05 was considered statistically significant. SAS statistical software (Version 9.1; SAS Institute Inc., Cary, NC) was used for the statistical analyses.
Results
The study population of 107 patients consisted of 47 male (44%) and 60 (56%) female subjects and their age range at the time of diagnosis was 23–90 years. Their mean age was 58.6 and median age 60.0 years. The shortest follow-up time was 0.1 years and longest 15.9 years. The follow-up time of patients who survived ranged from 10.6 to 15.9 years, and of patients who died from 0.1 to 15.7 years. Disease stage was as follows: stage I 67 patients, stage II 5 patients, stage III 7 patients and stage IV 24 patients. The TNM classification of four patients was unknown.
To standardize the staining sections a multibloc of normal salivary glands served as a control of the staining in each staining patches. A weak staining was seen in the epithelial cells of the salivary ducts and in some of the serous acinar cells (). Mucus secreting cells were negative for MMP-7.
Figure 1. Immunohistochemical stainings of MMP-7 in salivary gland cancer. The intensity of the cytoplasmic staining on a scale from 0 to 3 and the percentage of staining cells in parentheses. (A) Healthy salivary gland tissue. The intensity of epithelial cells stained is 1, some of the acinar cells are positive; (B) AdCC (3, 95%). Staining of nuclei is also visible; (C) AcCC (1, 40%). Some nuclei are stained; (D) MEC (2, 30%); (E) SDC (1, 30%). Scale bars: A, B, C, D, 100 μm.
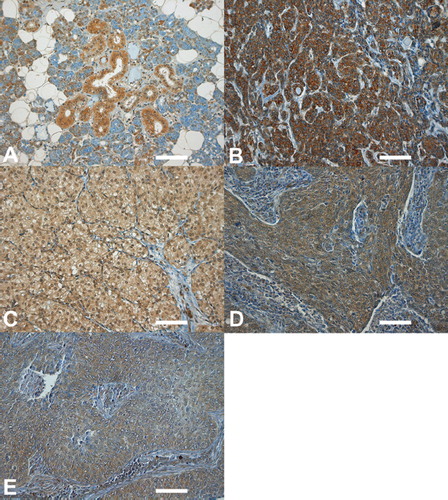
In the tumors MMP-7 stained was in general in the cytoplasma and varied from weak to strong (). The index of MMP-7 immunohistochemistry in the tumor tissue varied from 0 to 300. The mean value in MMP-7 was 75.6 (median 60). Immunohistochemical staining could not be used to differentiate the histologies of the tumors. The intensity, percentage and index are seen in . Staining intensity was lowest in salivary duct carcinoma (mean 1.2) and highest in adenoid cystic carcinoma (mean 1.7), whereas the percentage of the stained cells was lowest in acinic cell carcinoma (mean 34.6) and highest in salivary duct carcinoma (mean 44.2).
Table I. Immunohistochemical evaluation of MMP-7 in salivary gland cancer tissue. The intensity is evaluated on a scale of 0-3. Percent is the proportion of staining cells. The index is the product of staining intensity and percentage of positive cancer cells.
Higher T class predicted worse overall survival in age-adjusted analysis (2–4 vs. 1, mean survival time; 7.0 vs. 12.1 years; p = 0.004, HR 2.5, 95% CI 1.3–4.5). The MMP-7 staining intensity, the percent of staining cells and the index did not associate with overall survival in age-adjusted analysis ().
Table II. Age-adjusted Cox regression analysis of MMP-7 immunohistochemistry and overall survival of patients with salivary gland carcinoma.
Patients with adenoid cystic carcinoma had significantly worse overall survival by age-adjusted analysis compared to patients with acinic cell carcinoma (7.5 vs. 10.0 years, p = 0.019, HR 2.7, 95% CI 1.2–6.2). The same was true for the comparison between salivary duct carcinoma and acinic cell carcinoma (4.2 vs. 10.0 years, p = 0.042, HR 2.9, 95% CI 1.0–7.8) ().
Table III. Age-adjusted and multivariate Cox regression analysis of prognostic factors for overall survival of patients with in salivary gland carcinomas.
Lower staining intensity compared to higher staining intensity was associated with worse age-adjusted overall survival of patients with acinic cell carcinoma (0–1 vs. 2–3; p = 0.047, HR 6.5, 95% Cl 1.0–41. 7) and mucoepidermoid carcinoma (p = 0.010, HR 9.3, 95% CI 1.7–50.0). A low staining intensity was also associated with worse age-adjusted disease-specific survival of patients with acinic cell carcinoma (0–1 vs. 2–3; p = 0.047, HR 13.8, 95% CI 1.0–182.7). VCI Ki-67 was an important prognostic factor in the whole data set in age-adjusted overall (> 20 vs. ≤ 20, 5.7 vs. 11.9 years; p < 0.0001, HR 3.4, 95% Cl 2.0–6.0) and disease specific survival (> 20 vs. ≤ 20, 6.5 vs. 13.4 years; p < 0.0001, HR 4.7, 95% Cl 2.3–9.8).
T status, N status, stage, gender and diagnosis were significant explanatory variables in the age-adjusted models and were included in the multivariate Cox regression analysis (). In Cox multivariate regression analysis age, stage, gender, diagnosis and VCI Ki-67 were prognostic factors for overall survival. In disease-specific survival analysis lymph node (p = 0.037, HR 3.1, 95% CI 1.1–8.4), stage (p < 0.0001, HR 8.6, 95% CI 2.9–25.2), gender (p = 0.006, HR 3.0, 95% CI 1.4–6.5) and VCI Ki-67 (p = 0.017, HR 2.7, 95% CI 1.2–6.0) were significant prognostic factors. In contrast, MMP-1, -9, -13 did not associate significantly with age-adjusted overall or disease specific survival.
Discussion
Interestingly, high rather than low MMP-7 expression was associated with better survival of patients with acinic cell and mucoepidermoid carcinoma. To our knowledge, neither the expression of MMP-7 nor its association with survival of patients with SGC has been studied before. In previous studies involving patients with other solid tumors (pancreatic adenocarcinoma [Citation16] and esophageal squamous cell carcinoma [Citation17]) increased MMP-7 expression in cancer cells has been associated with a poor prognosis. The expression of MMP-7 has also been associated with recurrence of hepatocellular cancer [Citation18] and with lymph node metastasis of gastric carcinoma [Citation19]. An inverse association between MMP-7 expression and tumor size, N positivity, large pT and poor differentiation has been reported for papillary thyroid carcinoma [Citation20]. In our study lower staining intensity of MMP-7 was associated with poor overall survival of patients with acinic cell and mucoepidermoid carcinomas.
The diminished expression of MMP-7 in cancer cells may be caused by incomplete differentiation, since healthy salivary gland tissue also expresses MMP-7 [Citation9]. This observation is not surprising, since certain MMPs are tumor suppressive [Citation21].
MMP-7 is not expressed in healthy human skin keratinocytes, but is abundant in Bowen's disease and cutaneous squamous cell carcinoma (SCC); it is most abundant in poorly differentiated SCC. Thus, the expression of MMP-7 increases with the degree of malignancy [Citation22]. In the present study the expression of MMP-7 was weak in normal salivary gland tissue, as reported earlier by Saarialho-Kere [Citation9], but stronger in SGC, which conforms with the concept that malignant transformation in salivary gland tissue causes a similar change in the expression of MMP-7 as in skin cancer. Specific induction of MMP-7 expression in malignant salivary gland tissue may promote the aggressive biological behavior of SGC; MMP-7 could thus act as an early marker of SGC. This hypothesis raises the interesting prospect of screening and early detection of SGC by assessing MMP-7 in the saliva.
Several factors are involved in tumorigenesis and, of course, the MMPs do not operate in isolation. In murine mammary gland cell lines MMP-7 processes other growth factors into soluble forms; MMP-7 activates through proteolysis other proteinases that, in turn, promote cell proliferation. MMP-7 mediates the processing of ErbB4 involved in upstream MMP-7 activity in mammary gland tumorigenesis [Citation23].
The general belief is that overexpression of a certain MMP, either by tumor cells or by the surrounding stroma, leads to malignant transformation. Maybe the expression of certain MMPs, either at the primary or the metastatic site, promotes multiple stages of cancer progression by providing a pro-tumorigenic and protective milieu in the tissue [Citation24].
Mammary epithelial cells that are transiently exposed to MMP-7 undergo enhanced apoptosis, while cells that are subjected to continuous exposure and constant circulating levels of Fas ligand (FasL) show a 50% reduction in Fas-induced apoptosis [Citation25,Citation26].
In our previous study using the same cancer tissue material and patient population, high MMP-13 staining intensity predicted poor overall survival [Citation14]. Also, a high MMP-13 intensity percentage and index were associated with poor survival among patients with acinic cell carcinoma. Survival was also compromised for patients whose adenoid cystic carcinoma exhibited a high MMP-9 index and for patients whose salivary duct carcinoma had a high percentage of MMP-9 positive cells. In addition, a high MMP-1 staining intensity and index (% * intensity) is associated with longer overall survival in the whole data set [Citation14]. In the present study, survival was longer for patients with acinic cell and in mucoepidermoid carcinoma whose tumor expressed strongly MMP-7. By Cox multivariate regression analysis MMP-1, -9 and -13 did not associate significantly with survival, while VCI Ki-67 was a powerful prognostic factor.
Many variables need to be considered when evaluating the biological effects of matrilysin in SGC tissue. It is known that the quantity of proteolytically active enzyme capable of degrading the stromal matrix is determined by the molar balance between the nonspecific inhibitors in the serum (e.g., α1-proteinase inhibitor and α2-macroglobulin) and the active inhibitors of metalloproteinases in the cells and tissue. Similarly, the membrane anchored regulator of matrix metalloproteinases (RECK) inhibits tumor invasion and angiogenesis [Citation8].
Immunohistochemistry allows an immediate assessment of the expression intensity and extent in cell patterns and this facilitates prediction of the biological relevance of the immunohistochemical findings. MMP are expressed in cancer tissue and, to some extent, in the peritumoral area, preferentially in fibroblasts and inflammatory cells [Citation7,Citation8].
Although numerous factors influence the role and significance of matrilysin in the pathogenesis, growth, invasion and metastasis of cancer, the current study shows that lower MMP-7 staining intensity is associated with worse overall survival of patients with acinic cell and mucoepidermoid carcinoma. MMP-7 may be a useful prognostic factor for the assessment of patients with SGC.
Acknowledgements
We thank Ms Sinikka Kollanus and Sari Pitkänen for skillful technical assistance. This study was supported by grants from the Cancer Foundation of South-Western Finland, the Research Foundation of Orion Corporation and the Turku Municipal Health Department, Turku University Central Hospital (EVO 13011) and by grants from the Academy of Finland, the Finnish Cancer Research Foundation, the Sigrid Juselius Foundation and the Turku University Hospital (13336) to V.-M.K. A.K. is a student of the National Graduate School of Clinical Medicine. The authors thank the University Hospitals and the Central Hospitals of Finland for providing data to this study.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Shah JP, Patel SG. Head and neck surgery and oncology. 3rd. Edinburgh: Mosby, 2003.
- Östman J, Anneroth G, Gustafsson H, Tavelin B. Malignant salivary gland tumours in Sweden 1960–1989--an epidemiological study. Oral Oncol 1997;33:169–76.
- Finnish Cancer Registry. October 10th 2008. Available from: URL:http://www.cancerregistry.fi/eng/.
- Luukkaa H, Klemi P, Leivo I, Koivunen P, Laranne J, Mäkitie A, . Salivary gland cancer in Finland 1991–96: An evaluation of 237 cases. Acta Otolaryngol 2005;125:207–14.
- Chen W, Zhang HL, Shao XJ, Jiang YG, Zhao XG, Gao X, . Gene expression profile of salivary adenoid cystic carcinoma associated with perineural invasion. Tohoku J Exp Med 2007;212:319–34.
- De S, Fenton JE, Jones AS. Matrix metalloproteinases and their inhibitors in non-neoplastic otorhinolaryngological disease. J Laryngol Otol 2005;119:436–42.
- Ala-aho R, Kähäri VM. Collagenases in cancer. Biochimie 2005;87:273–86.
- Vihinen P, Ala-aho R, Kähäri VM. Matrix metalloproteinases as therapeutic targets in cancer. Curr Cancer Drug Targets 2005;5:203–20.
- Saarialho-Kere UK, Crouch EC, Parks WC. Matrix metalloproteinase matrilysin is constitutively expressed in adult human exocrine epithelium. J Invest Dermatol 1995;105:190–6.
- Johansson N, Kähäri VM. Matrix metalloproteinases in squamous cell carcinoma. Histol Histopathol 2000;15:225–37.
- Wilson CL, Ouellette AJ, Satchell DP, Ayabe T, Lopez-Boado YS, Stratman JL, . Regulation of intestinal alpha-defensin activation by the metalloproteinase matrilysin in innate host defense. Science 1999;286(5437):113–7.
- Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function, and biochemistry. Circ Res 2003;92:827–39.
- Pozzi A, Moberg PE, Miles LA, Wagner S, Soloway P, Gardner HA. Elevated matrix metalloprotease and angiostatin levels in integrin alpha 1 knockout mice cause reduced tumor vascularization. Proc Natl Acad Sci US 2000;97:2202–7.
- Luukkaa H, Klemi P, Hirsimaki P, Vahlberg T, Kivisaari A, Kähäri VM, . Matrix metalloproteinase (MMP)-1, -9 and -13 as prognostic factors in salivary gland cancer. Acta Otolaryngol 2008;128:482–90.
- Luukkaa H, Klemi P, Leivo I, Vahlberg T, Grenman R. Prognostic significance of Ki-67 and p53 as tumor markers in salivary gland malignancies in Finland: An evaluation of 212 cases. Acta Oncol 2006;45:669–75.
- Yamamoto H, Itoh F, Iku S, Adachi Y, Fukushima H, Sasaki S, . Expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in human pancreatic adenocarcinomas: Clinicopathologic and prognostic significance of matrilysin expression. J Clin Oncol 2001;19:1118–27.
- Yamashita K, Mori M, Shiraishi T, Shibuta K, Sugimachi K. Clinical significance of matrix metalloproteinase-7 expression in esophageal carcinoma. Clin Cancer Res 2000;6:1169–74.
- Yamamoto H, Itoh F, Adachi Y, Fukushima H, Itoh H, Sasaki S, . Messenger RNA expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in human hepatocellular carcinoma. Jpn J Clin Oncol 1999;29:58–62.
- Yamashita K, Azumano I, Mai M, Okada Y. Expression and tissue localization of matrix metalloproteinase 7 (matrilysin) in human gastric carcinomas. Implications for vessel invasion and metastasis. Int J Cancer 1998;79:187–94.
- Ito Y, Yoshida H, Kakudo K, Nakamura Y, Kuma K, Miyauchi A. Inverse relationships between the expression of MMP-7 and MMP-11 and predictors of poor prognosis of papillary thyroid carcinoma. Pathology 2006;38:421–5.
- Lopez-Otin C, Matrisian LM. Emerging roles of proteases in tumour suppression. Nat Rev Cancer 2007;7:800–8.
- Kivisaari AK, Kallajoki M, Mirtti T, McGrath JA, Bauer JW, Weber F, . Transformation-specific matrix metalloproteinases (MMP)-7 and MMP-13 are expressed by tumour cells in epidermolysis bullosa-associated squamous cell carcinomas. Br J Dermatol 2008;158:778–85.
- Lynch CC, Vargo-Gogola T, Martin MD, Fingleton B, Crawford HC, Matrisian LM. Matrix metalloproteinase 7 mediates mammary epithelial cell tumorigenesis through the ErbB4 receptor. Cancer Res 2007;67:6760–7.
- Martin MD, Matrisian LM. The other side of MMPs: Protective roles in tumor progression. Cancer Metastasis Rev 2007;26:717–24.
- Fingleton B, Vargo-Gogola T, Crawford HC, Matrisian LM. Matrilysin [MMP-7] expression selects for cells with reduced sensitivity to apoptosis. Neoplasia 2001;3:459–68.
- Vargo-Gogola T, Crawford HC, Fingleton B, Matrisian LM. Identification of novel matrix metalloproteinase-7 (matrilysin) cleavage sites in murine and human Fas ligand. Arch Biochem Biophys 2002;408:155–61.
