Abstract
Pancreatic cancer remains a deadly disease. Despite advances on many fronts, surgeons play a leading role in the diagnosis and management of pancreatic cancer. Preoperative staging is best provided by “pancreas-protocol” abdominal CT, although endoscopic ultrasound and diagnostic laparoscopy can add value in selected patients. Surgical resection, which remains the only curative option, is now accomplished with uniformly low perioperative mortality in high-volume centers (<3%), although complications remain frequent. Unfortunately, the long-term prognosis for pancreatic cancer remains poor with 5-year survival rates only 15–23% with median survival of 13 to 18 months. Recent data from randomized trials have supported the role for adjuvant chemotherapy and questioned the traditional role of radiation. Early diagnosis and targeted multimodality treatments would appear essential to optimizing the results of surgical therapy.
Pancreatic adenocarcinoma is one of the most deadly malignancies worldwide. In the United States alone, it is estimated that 42 470 new cases would be diagnosed in 2009, and an estimated 35 240 would die that same year [Citation1]. Resistance to both chemotherapy and radiation [Citation2] has rendered pancreatic cancer largely a surgical disease. The role of the surgeon in offering complete resection remains pivotal and currently the only potentially curative option. Unfortunately, only 15–20% of patients diagnosed with pancreatic adenocarcinoma are candidates for resection, and even after complete (R0) resection the 5-year survival is only 15–20% [Citation3,Citation4]. This poor prognosis, attributable to delayed presentation, tumor biology, complexity of surgical intervention, and paucity of multimodality therapy, has engrained a pessimistic mindset in many clinicians, leading to an underutilization of surgery despite recent evidence challenging these long-established beliefs [Citation5].
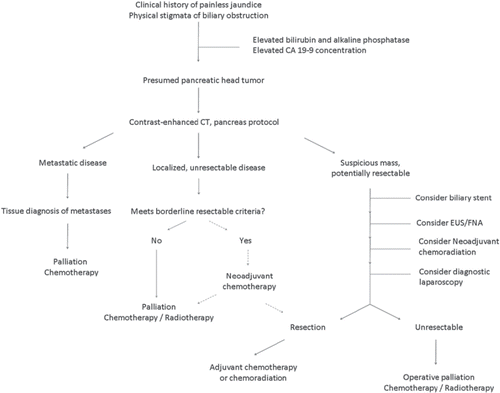
Progress in the last decade has specifically shown decreased peri-operative mortality and acceptable complication rates after surgical resection [Citation3,Citation4]. These advances have been attributed to a shift of complex surgery to regional and referral centers [Citation6]. This manuscript will briefly review pathology and presentation of patients with pancreatic adenocarcinoma and focus on the current management of this disease ().
Pathology
Tumors of the pancreas can arise from many pancreatic cell lines, and are classified according to gross appearance, hormonal function, and the cell type of origin. Ductal epithelium gives rise to at least 75% of all non-functioning pancreatic cancer, despite comprising a disproportionately small component (<10%) of the pancreatic cell volume. Current thinking suggests that pancreatic adenocarcinoma arises from precursor proliferations of the ductal epithelium, also termed intraepithelial neoplasia, which accumulate genetic changes as they propagate [Citation7]. Recent evidence further suggests that dedifferentiation of acinar cells to epithelial cells may contribute to pancreatic carcinogenesis [Citation8]. A number of variants of pancreatic ductal adenocarcinoma exist, including adenosquamous carcinoma and acinar cell carcinoma. These make up a minority of pancreatic malignancies and behave comparably to pancreatic adenocarcinoma.
It is estimated that 65% of pancreatic adenocarcinomas arise in the pancreatic head, 15% in body and tail, and 20% involve the gland diffusely. The location of the tumor contributes to the clinical picture. Carcinoma of pancreatic head obstructs the common bile duct and leads to jaundice and chronic obstructive pancreatitis. Pancreatic adenocarcinoma is aggressive, invading surrounding organs and infiltrating into vasculature, lymphatics and the perineural sheath. Studies in the last decade have shown that lymphatic and perinerual invasion are independent prognostic factors of poor survival [Citation3,Citation4]. Metastasis to the liver, peritoneal cavity, lungs and invasion of major visceral vessels represent evidence of advanced disease and preclude surgical resection.
Cystic pancreatic lesions may be benign pseudocysts related to prior pancreatitis or true cystic neoplasms. Many cystic tumors are asymptomatic and are being recognized with increasing frequency owing to widespread use of abdominal imaging for other reasons [Citation9]. Two forms of cystic neoplasms have clear malignant potential – mucinous cystadenomas and intraductal papillary mucinous neoplasms (IPMN). The management of benign IPMN is complex due to their malignant potential and is dictated by symptoms and tumor size [Citation10].
Many factors influence the epidemiology of pancreatic cancer; the most prominent of these are increasing age and tobacco smoke. Most cases of pancreatic cancer are sporadic, with only approximately 10% following a hereditary pattern [Citation11]. Recent progress in molecular genetics has uncovered avenues for novel identification and screening factors in persons at risk [Citation11].
Diagnosis and clinical staging
Clinical presentation
Early diagnosis of pancreatic cancer is difficult because of non-specific symptomatology and inaccessibility of the pancreas to clinical exam. Biliary obstruction manifesting as jaundice is the most common presentation, and is accompanied by dark urine, acholic stools and pruritis. Most clinicians adhere to the dogma that painless jaundice after the 5th or 6th decaude of life is pancreatic cancer until proven otherwise. Vague abdominal pain relates to ductal obstruction or invasion of the neural plexus. Back pain is an ominous sign. Recent data suggest a temporal relationship between new-onset diabetes mellitus as a harbringer of pancreatic malignancy [Citation12], suggesting that this manifestation could guide the identification of asymptomatic and early-stage pancreatic cancer. The physical findings of pancreatic cancer include jaundice, a palpable gallbladder, hepatomegaly, and ascites in advanced cases. Peripheral adenopathy usually signifies advanced disease and portends a poor prognosis.
Laboratory workup
Biliary obstruction is reflected by an elevation of serum bilirubin and alkaline phosphatase. The absence of bile salts due to biliary obstruction can result in a prolonged international normalized ratio (INR) due to malabsorption of vitamin K. Malnutrition develops from fat and protein malabsorption and tumor cachexia and is reflected by hypoalbuminemia.
Carbohydrate antigen 19-9 (CA 19-9) is a tumor marker that has a specificity greater than 95% for pancreatic adenocarcinoma when serum concentration is >200 U/mL [Citation13]. This marker is useful for both diagnostic and follow-up after therapy. Of note, CA19-9 is associated with the Lewis Antigen (Ag), and therefore the 5% of the population who do not produce Lewis Ag will not manifest elevation of CA 19-9 even in the face of widespread disease [Citation13].
Radiologic imaging
Non-invasive imaging is the mainstay for both the diagnosis and staging of pancreatic cancer. Transabdominal ultrasonography is inexpensive and rapid, but is operator dependent. It is most useful in demonstrating a dilated biliary tree in jaundiced patients but is otherwise of limited value in patients with pancreatic cancer [Citation14].
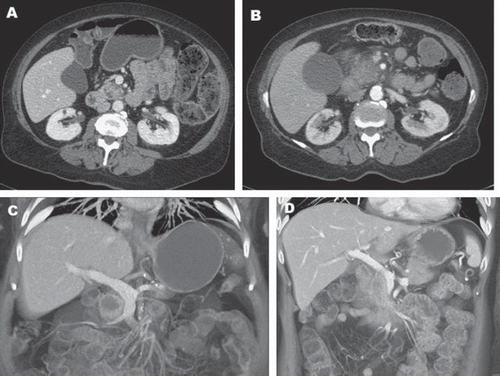
Abdominal computed tomography (CT), with specific “pancreas protocol” contrast administration to depict both arterial and venous phases, is the modality of choice for identifying pancreatic tumors and hepatic metastases (). Resectibility can be predicted by contrast CT in up to 80% of patients in the absence of extrapancreatic disease, a patent superior mesenteric-portal venous confluence, and absence of invasion into the celiac axis or superior mesenteric artery (SMA) [Citation14].
Figure 2. Contrast-enhanced CT of the abdomen showing evidence of a hypodense mass in the uncinate process. Patient A has a clearly defined fat plane between the mass and the superior mesenteric vein (SMV). The fat plane is absent in Patient B suggesting invasion of the vein. C and D show coronal views of a mass without and with SMV invasion, respectively.
Magnetic resonance imaging (MRI) of the abdomen is more costly than CT imaging and suffers from motion artifact and lack of bowel opacification. Generally, MRI offers little advantage over CT scanning for the diagnosis of pancreatic cancer, though the role and indications for magnetic resonance cholangiopancreaticography (MRCP) continue to expand. Magnetic resonance imaging with MRCP is currently indicated for non-invasive diagnostic imaging to evaluate the biliary and pancreatic ducts and may be the optimal method to survey patients with IPMN and the pancreatic remnant after surgery [Citation10].
Positron emission tomography (PET) scanning has a developing role in the evaluation of pancreatic cancer. Current data suggests that PET may change the clinical management in up to 40% of patients [Citation15]. Major drawbacks of PET include high cost and long study time, as well as decreased specificity in the face of inflammation.
Endoscopic evaluation
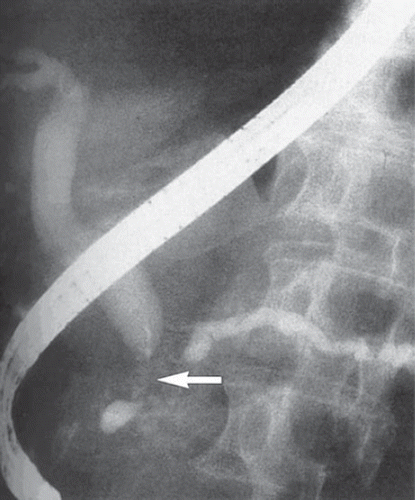
Endoscopic retrograde cholangiopancreaticography (ERCP) historically served both a diagnostic and therapeutic role in patients with obstructive jaundice due to periampullary cancer. The diagnostic function of ERCP has slowly been replaced by MRCP circumventing the need for an invasive procedure (). Current application of ERCP in pancreatic cancer has shifted towards therapeutic and palliative stenting [Citation16].
Figure 3. ERCP demonstrating a classic “double duct” sign. The arrow shows tumor obstruction of both the bile duct (left) and pancreatic duct (right).
The use of endoscopic ultrasound (EUS) is gaining popularity in the diagnosis and staging of pancreatic tumors. Recent studies show that EUS is complementary to CT both for identification of the primary tumor and extension into major visceral vessels [Citation17]. Endoscopic ultrasound offers the ability to perform fine needle aspiration (FNA) to provide a cytologic diagnosis, which is 80–90% sensitive and nearly 100% specific for diagnosing pancreatic adenocarcinoma. Endoscopic ultrasound may be superior to CT at identifying tumors and metastatic implants <1 cm in size [Citation18].
Resection of pancreatic cancer
Walter Kausch first performed a pancreaticoduodenal resection in 1912 [Citation19], however, the momentum for pancreatic resection is largely attributable to Allan O. Whipple’s historic work in the late 1930s [Citation20]. For the better part of the 20th century, pancreatic resection was associated with significant morbidity and mortality, leading some to condemn the procedure in the 1970s [Citation21]. In the late 1980s and early 1990s, however, a handful of high volume centers reported marked improvement in perioperative mortality, including two large series in excess of 100 patients without a mortality [Citation22,Citation23]. These improved outcomes have led to a more aggressive attitude at most academic centers. Unfortunately, this attitude is not universal with significant nihlism still remaining [Citation5].
Diagnostic laparoscopy
Even with considerable improvement in diagnostic imaging, metastatic disease may manifest as subcentimeter liver and peritoneal deposits that are identified in 10–20% of patients only at the time of operative exploration [Citation24]. Laparoscopy can identify such small volume disease via minimal invasive techniques [Citation24]. Some surgeons recommend diagnostic laparoscopy when CA19-9 levels are extremely high or patients have clinical and radiologic evidence suggesting advanced disease [Citation24].
Proximal pancreatic resection
Thorough abdominal exploration is a critical first operative step, as identification of distant metastatic disease contraindicates resection. After excluding distant disease, tumor involvement of the major visceral vessels is evaluated. Criteria for unresectability include occlusion of the portal or superior mesenteric vein (SMV), or direct involvement of the SMA, hepatic artery or celiac axis. Most experienced pancreatic surgeons will proceed with resection without a histologic diagnosis.
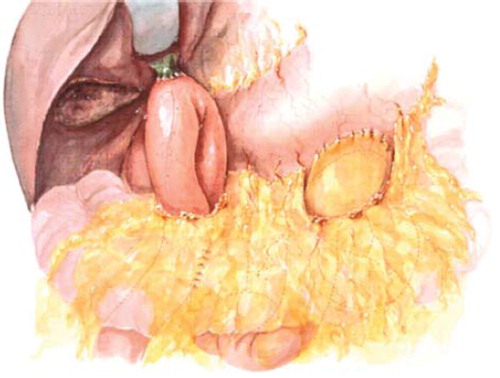
A number of variations on resection and reconstruction exist, and are applied based on individual patient and tumor characteristics. The pylorus- preserving modification of pancreaticoduodenectomy (PD) is widely utilized (). This technique has been shown to improve post-operative gastrointestinal function [Citation25], with no difference in long-term survival when compared to a classic PD [Citation26]. Japanese and Italian data initially suggested improved survival with extended lymphadenectomy with dissection of the celiac and peri-aortic nodes [Citation27,Citation28]. However, this survival advantage was not reproduced in two American studies [Citation29,Citation30]. Extended lymphadenectomy is associated with increased morbidity, and in some studies, with increased mortality, and therefore is not routinely performed by Western surgeons.
Figure 4. Diagram of the resection (A) and final reconstruction (B) after pylorus-sparing pancreaticoduodenectomy. (Adapted from Cameron JL, Sandone C. Atlas of Gastointestinal Surgery, 2md ed. Vol 1. Hamilton, Ontario: BC Decker, Inc.; 2007:302.)
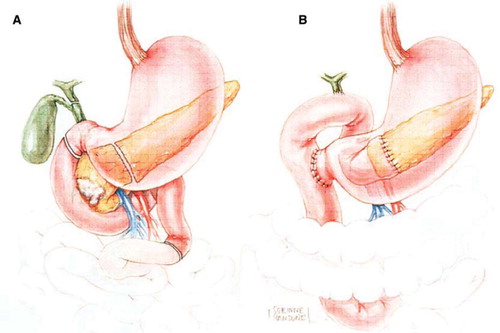
Total pancreatectomy is reserved for patients with IPMN involving the entirety of the pancreatic duct or for those with adenocarcinoma in whom a negative margin cannot be obtained on frozen section [Citation31]. Finally, laparoscopic pancreaticoduodenectomy has been reported by a few centers with highly skilled laparoscopic surgeons. This practice requires two discrete skill sets: expertise in pancreatic surgery and advanced laparoscopic skills.
Variations in the technique of reconstruction after PD have been employed in attempts to reduce the troublesome complications of pancreatic anastomotic leak and delayed gastric emptying. Many of these have been studied by prospective randomized trials, but, in general, most experienced pancreatic surgeons develop specific techniques based on personal experience.
Distal pancreatectomy
Many patients with pancreatic body and tail tumors are found to have unresectable disease due to delayed presentation and diagnosis. Therefore, the role of distal resection is limited. As with pancreaticoduodenectomy, the surgeon must be cognizant of intra-operative evidence of unresectibility. Extended lymphadenectomy increases morbidity and does not offer any survival advantage [Citation32]. No technique has been demonstrated to be superior in reducing the rate of fistula from the proximal remnant.
Laparoscopic distal pancreatectomy has been shown by mounting data to be appropriate for benign and low grade malignant tumors [Citation33]. Long-term outcomes after laparoscopic distal resection for adenocarcinoma have not been reported; this practice should be restricted to clinical trials as a potential option in the hands of skilled laparoscopic surgeons at high-volume centers.
Outcomes after resection
The most significant advance in surgical resection for pancreatic cancer is a substantial reduction in peri-operative mortality [Citation3,Citation4,Citation21]. The improved outcomes can be attributed to the evolution of operative technique and peri-operative care as well as the accumulation of surgical experience at high-volume pancreatic centers [Citation6].
Despite substantial decrease in mortality, the morbidity after PD remains as high as 30–40% (). Pancreatic fistula continues to haunt surgeons with incidences of 5–15% after proximal resection [Citation3,Citation5,Citation32]. Several factors including quality of the pancreatic parenchyma play a role in predicting the development of a pancreatic fistula [Citation34]. Multiple attempts at refining techniques to reduce the incidence of pancreatic fistula have been subjected to prospective randomized controlled trials. Specific examples include variations in operative and anastomotic technique, the use of pancreatic stents, somatostatin analogs [Citation35,Citation36] and fibrin glue [Citation37]. The level 1 studies have generated mixed results and therefore no definitive practice guidelines currently exist. Hemorrhage, though infrequent, is a potentially life-threatening complication of pancreatic resection that may occur either intraoperatively, in the early postoperative course, or later (in the background of pancreatic fistula). Early post-operative hemorrhage may be treated by re-exploration, while hemorrhage in the late post-operative period is best treated by interventional radiology.
Table I. Outcomes of pancreatic resection
Delayed gastric emptying plagues 10–20% of patients after PD. The understanding of this phenomenon is incomplete, but some improvement has been noted with the prokinetic drugs erythromycin and metoclopramide [Citation38]. Data evaluating the technique used for reconstruction suggests superiority of the antecolic versus retrocolic duodenojejunostomy [Citation39].
Distal pancreatectomy is associated with a periperative mortality of 2 to 5% [Citation33,Citation40]. Complications are less frequent that following PD, although pancreatic fistula rates are often higher at 5 to 30%.
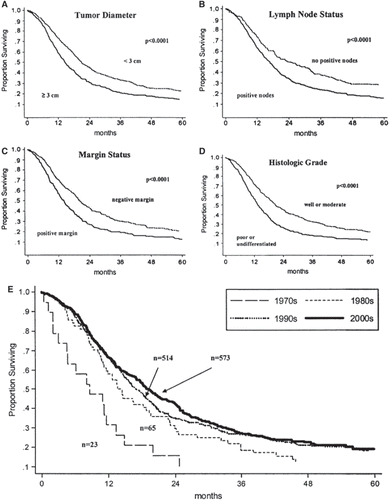
The long-term survival following resection of pancreatic cancer remains disappointing (). Current five year survival rates show ranges from 15 to 23% with median survival of 13.6 to 17.4 months [Citation41]. Unfortunately, survival continues to fall even after the five years. Survival is improved by R0 resection, resection in high-volume centers, early tumor stage, and adenocarcinoma associated with IPMN [Citation31,Citation41].
Figure 5. Survival of patients with pancreaticoduodenectomy based on tumor size (A), lymph node status (B), margin status (C), histologic grade (D), and historical context (E). Reprinted with permission from: Winter JM, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: A single-institution experience. J Gastrointest Surg. 2006 Nov;10(9):1199–210.
Role of multimodality treatment
The multimodality approach to pancreatic cancer therapy has improved survival and has seen increased utilization over time [Citation42]. Options include preoperative chemoradiation, particularly for borderline resectable pancreatic cancer, in hope to “down-stage” tumors to improve resectability. In the postoperative setting either systemic chemotherapy or chemoradiation is routinely employed. Unfortunately, an optimized and widely adopted algorithm does not exist.
The high recurrence rate of pancreatic malignancy after potentially curative resection suggests that surgical intervention alone is not adequate even for patients with localized, resectable tumors. Therefore, adjuvant therapy with chemotherapy and radiation, alone or in combination have been studied. External beam radiotherapy was originally used to treat locally-advanced non-resectable tumors, but had extremely high failure rates [Citation43]. The poor response to radiotherapy alone prompted the use of various chemotherapeutic agents as radio-sensitizers.
Inspired by experience with other gastrointestinal malignancies, adjuvant therapy with 5-fluorouracil (5FU) combined with radiation demonstrated a survival benefit in a study by the Gastrointestinal Tumor Study Group (GITSG) in the mid-1980s [Citation44]. More recently, however, the European Study Group for Pancreatic Cancer (ESPAC) demonstrated significantly greater 2-year and 5-year survival rates in patients who received postoperative chemotherapy with 5FU compared with patients who received no chemotherapy [Citation2,Citation45], whereas 5FU with radiation therapy showed no benefit and in fact worse survival. The Eastern Cooperative Oncology Group (ECOG) has also shown mixed survival benefits with several 5FU-based protocols alone and in combination with radiotherapy [Citation46,Citation47]. The data from these and subsequent studies has largely manifested into a dichotomy between the European practice, largely excluding chemoradiation based on the ESPAC studies, and the North American approach, employing chemoradiotherapy because of the high risk of local failure.
In the last decade, gemcitabine has also proved to be a potent radiosensitizer. Gemcitabine-based chemoradiotherapy has shown a survival advantage over both placebo for adjuvant therapy and 5FU [Citation48]. Gemcitabine-based therapy in patients with unresectable pancreatic cancer is therefore considered by many to be the systemic modality of choice for pancreatic cancer [Citation49]. Several newer agents have been investigated in combination with gemcitabine including capecitabine and docetaxel, however, the benefit over gemcitabine monotherapy has been minimal and not uniformly reproducible.
Unfortunately, the ultimate benefit conferred by adjuvant chemotherapy with or without radiotherapy are modest. Ongoing research has proposed novel targets and agents as directions toward multimodality therapy. One interferon-based protocol described by Picozzi et al showed improved overall survival but is associated with high toxicity [Citation50]. Attempts at reproducing these results have been mixed [Citation51].
The difficult problem of locally advanced borderline resectable pancreatic cancer has stimulated investigation of preoperative neoadjuvant therapy [Citation52]. Neoadjuvant therapy has several theoretical advantages, including selecting for tumor biology, downstaging the primary tumor and improving the rate of R0 resection [Citation53]. A number of trials, combining chemotherapy and radiotherapy and subsequently increasing the dosing and intensity of the regimens, have been attempted. Unfortunately, none of these trials demonstrate an obvious improvement in either resectability or overall survival [Citation54,Citation55]. Nevertheless, the accumulation of this data would appear to support an initial period of neoadjuvant chemotherapy for patients with locally advanced or borderline resectable pancreatic cancer. Patients should then be restaged with imaging by CT and considered for either resection or subsequent chemoradiotherapy [Citation56].
The median survival for patients with Stage IV pancreatic cancer is three to six months. Studies of single-agent chemotherapy for metastatic pancreatic cancer have shown modest objective response and clinical benefit, including symptom relief and performance status. Gemcitabine monotherapy has been shown to have better clinical benefit and survival than 5FU [Citation49]. Capecitabine was shown to have an objective response rate in 7% of patients and clinical benefit in 24% of patients in a Phase II trial [Citation57]. A number of studies using multiagent therapy have failed to show clear survival benefit in this setting. The lack of substantial survival benefit for Stage IV disease should lead to frank discussions of quality of life issues with the patient, as well as focus on individual patients’ specific goals.
Palliation
Since most patients with pancreatic cancer present with unresectable disease, symptomatic palliation (jaundice, pain, gastric outlet obstruction) is an important therapeutic focus. Obstructive jaundice occurs in as many as 80% of patients with adenocarcinoma of the pancreatic head. Cholestasis manifests with debilitating pruritis and may eventually progress to hepatic failure and premature death if not addressed promptly. Several randomized trials have compared surgical biliary bypass to endoscopic palliation in patients with obstructive jaundice due to pancreatic cancer [Citation58,Citation59]. In general, both surgical and endoscopic approaches offer comparable efficacy, though endoscopic stenting significantly reduces length of hospital stay. The development of large- caliber endobiliary metallic stents has offered improved long-term patency of endoscopic palliation.
Gastric outlet obstruction is less common, but has been described in up to 20% of patients, regardless of location of the tumor. Nausea and emesis in patients with pancreatic adenocarcinoma is not necessarily related to mechanical gastric outlet obstruction, as the etiology of these symptoms in the setting of pancreatic malignancy is complex and likely involves neural invasion by the tumor and subsequent dysmotility. Prospective randomized studies have demonstrated that prophylactic gastrojejunostomy in patients without pre-operative symptoms but found to be unresectable during surgical exploration reduces subsequent gastric outlet obstruction [Citation60,Citation61]. For patients with gastric outlet obstruction in the face of inoperable disease, treatment with endoscopically-placed metallic stents has become the standard of care.
Pain due to pancreatic cancer is a manifestation of both neural invasion and obstructive ductal physiology; pain is present in essentially all patients to some extent. Intervention in the form of a celiac neurolysis with chemical agents decreases pain [Citation62], and may actually improve survival. Celiac neurolysis may be performed intraoperatively, percutaneously or endoscopically under US guidance [Citation63,Citation64].
Improved endoscopic techniques addressing both biliary and duodenal obstruction have largely eliminated the use of operation solely for palliation in most patients. Nevertheless, surgical hepaticojejunostomy and gastrojejunostomy provide durable long-term palliation with acceptable perioperative morbidity and mortality and are still considered standard of care when a patient is found intraoperatively to have locally advanced unresectable disease (). Surgical biliary and gastric bypass procedures may be performed laparoscopically by skilled minimally invasive surgeons.
Conclusion
The modern era has witnessed great progress coupled with gradually evolving attitudes toward the surgical intervention for pancreatic cancer. Undoubtedly, these advances have reinforced the role of the surgeon in both curative resection and palliation. However, despite greatly reduced perioperative mortality with pancreatic resection, morbidity remains high, and the overall survival of pancreatic cancer patients, even after R0 resection, is dismal. Surgeons, both as scientists and clinicians, must continue to investigate new approaches focusing on improving the early diagnosis and novel, targeted multimodality treatments to further impact the outcomes of this deadly disease.
Acknowledgements
Presented as the Acta Oncologica Lecture at the XL Nordic Meeting of Gastroenterology.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- American Cancer Society. Cancer Statistics, 2009. CA Cancer J Clin 2009;59:225–49.
- Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, European Study Group for Pancreatic Cancer. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 2004;350:1200–10.
- Cameron JL, Riall TS, Coleman J, Belcher KA. One thousand consecutive pancreaticoduodenectomies. Ann Surg 2006; 244:10–5.
- Schmidt CM, Powell ES, Yiannoutsos CT, Howard TJ, Wiebke EA, Wiesenauer CA, . Pancreaticoduodenectomy: A 20-year experience in 516 patients. Arch Surg 2004;139: 718–25.
- Bilimoria KY, Bentrem DJ, Ko CY, Stewart AK, Winchester DP, Talamonti MS. National failure to operate on early stage pancreatic cancer. Ann Surg. 2007;246:173–80.
- Birkmeyer JD, Stukel TA, Siewers AE, Goodney PP, Wennberg DE, Lucas FL. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:2117–27.
- Hruban RH, Goggins M, Parsons J, Kern SE. Progression model for pancreatic cancer. Clin Cancer Res. 2000;6:2969–72.
- Zhu L, Shi G, Schmidt CM, Hruban RH, Konieczny SF. Acinar cells contribute to the molecular heterogeneity of pancreatic intraepithelial neoplasia. Am J Pathol 2007;171: 263–73.
- Spinelli KS, Fromwiller TE, Daniel RA, Kiely JM, Nakeeb A, Komorowski RA, . Cystic pancreatic neoplasms: Observe or operate. Ann Surg 2004;239:651–7; Discussion 657–9.
- Schmidt CM, White PB, Waters JA, Yiannoutsos CT, Cummings OW, Baker M, . Intraductal papillary mucinous neoplasms: Predictors of malignant and invasive pathology. Ann Surg. 2007;246:644–51.
- Canto MI. Screening and surveillance approaches in familial pancreatic cancer. Gastrointest Endosc Clin N Am 2008;18: 535–53, x.
- Chari ST, Leibson CL, Rabe KG, Timmons LJ, Ransom J, de Andrade M, . Pancreatic cancer-associated diabetes mellitus: Prevalence and temporal association with diagnosis of cancer. Gastroenterology. 2008;134:95–101.
- Ritts RE, Pitt HA. CA 19-9 in pancreatic cancer. Surg Oncol Clin N Am. 1998;7:93–101.
- Tamm EP, Bhosale PR, Lee JH. Pancreatic ductal adenocarcinoma: Ultrasound, computed tomography, and magnetic resonance imaging features. Semin Ultrasound CT MR. 2007;28:330–8.
- Heinrich S, Goerres GW, Schäfer M, Sagmeister M, Bauerfeind P, Pestalozzi BC, . Positron emission tomography/computed tomography influences on the management of resectable pancreatic cancer and its cost-effectiveness. Ann Surg. 2005;242:235–43.
- Pisters PW, Hudec WA, Hess KR, Lee JE, Vauthey JN, Lahoti S, . Effect of preoperative biliary decompression on pancreaticoduodenectomy-associated morbidity in 300 consecutive patients. Ann Surg. 2001;234:47–55.
- Dewitt J, Devereaux BM, Lehman GA, Sherman S, Imperiale TF. Comparison of endoscopic ultrasound and computed tomography for the preoperative evaluation of pancreatic cancer: A systematic review. Clin Gastroenterol Hepatol. 2006;4:717–25.
- Legmann P, Vignaux O, Dousset B, Baraza AJ, Palazzo L, Dumontier I, . Pancreatic tumors: Comparison of dual-phase helical CT and endoscopic sonography. AJR Am J Roentgenol. 1998;170:1315–22.
- Kausch W. Das Carcinom der Papilla Duodeni and seine radikale Entfeinung. Beitrage zur Klinishe Chirurgie 1912;78: 439–86.
- Whipple AO, Parsons WB, Mullins CR. Treatment of carcinoma of the Ampulla of Vater. Ann Surg. 1935;102:763–79.
- Crile G Jr. The advantages of bypass operations over radical pancreatoduodenectomy in the treatment of pancreatic carcinoma. Surg Gynecol Obstet. 1970;130:1049–53.
- Trede M, Schwall G, Saeger HD. Survival after pancreatoduodenectomy. 118 consecutive resections without an operative mortality. Ann Surg. 1990;211:447–58.
- Cameron JL, Pitt HA, Yeo CJ, Lillemoe KD, Kaufman HS, Coleman J. One hundred and forty-five consecutive pancreaticoduodenectomies without mortality. Ann Surg. 1993;217:430–5.
- Conlon KC, Dougherty E, Klimstra DS, Coit DG, Turnbull AD, Brennan MF. The value of minimal access surgery in the staging of patients with potentially resectable peripancreatic malignancy. Ann Surg. 1996;223:134–40.
- Traverso LW, Longmire WP Jr. Preservation of the pylorus in pancreaticoduodenectomy. Surg Gynecol Obstet 1978; 146:959–62.
- Bell RH Jr. Pancreaticoduodenectomy with or without pylorus preservation have similar outcomes. Cancer Treat Rev. 2005;31:328–31.
- Satake K, Nishiwaki H, Yokomatsu H, Kawazoe Y, Kim K, Haku A, . Surgical curability and prognosis for standard versus extended resection for T1 carcinoma of the pancreas. Surg Gynecol Obstet. 1992;175:259–65.
- Pedrazzoli S, DiCarlo V, Dionigi R, Mosca F, Pederzoli P, Pasquali C, . Standard versus extended lymphadenectomy associated with pancreatoduodenectomy in the surgical treatment of adenocarcinoma of the head of the pancreas: A multicenter, prospective, randomized study. Lymphadenectomy Study Group. Ann Surg 1998;228: 508–17.
- Riall TS, Cameron JL, Lillemoe KD, Campbell KA, Sauter PK, Coleman J, . Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma– part 3: Update on 5-year survival. J Gastrointest Surg 2005;9: 1191–204.
- Farnell MB, Pearson RK, Sarr MG, DiMagno EP, Burgart LJ, Dahl TR, Pancreas Cancer Working Group. A prospective randomized trial comparing standard pancreatoduodenectomy with pancreatoduodenectomy with extended lymphadenectomy in resectable pancreatic head adenocarcinoma. Surgery 2005;138:618–28; Discussion 628–30.
- Schmidt CM, Glant J, Winter JM, Kennard J, Dixon J, Zhao Q, . Total pancreatectomy (R0 resection) improves survival over subtotal pancreatectomy in isolated neck margin positive pancreatic adenocarcinoma. Surgery. 2007;142:572–8.
- Strasberg SM, Drebin JA, Linehan D. Radical antegrade modular pancreatosplenectomy. Surgery. 2003;133:521–7.
- Kooby DA, Gillespie T, Bentrem D, Nakeeb A, Schmidt MC, Merchant NB, . Left-sided pancreatectomy: A multicenter comparison of laparoscopic and open approaches. Ann Surg. 2008;248:438–46.
- Mathur A, Pitt HA, Marine M, Saxena R, Schmidt CM, Howard TJ, . Fatty pancreas: A factor in postoperative pancreatic fistula. Ann Surg. 2007;246:1058–64.
- Sarr MG. The potent somatostatin analogue vapreotide does not decrease pancreas-specific complications after elective pancreatectomy: A prospective, multicenter, double-blinded, randomized, placebo-controlled trial. J Am Coll Surg. 2003;196:556–64.
- Yeo CJ, Cameron JL, Lillemoe KD, Sauter PK, Coleman J, Sohn TA, . Does prophylactic octreotide decrease the rates of pancreatic fistula and other complications after pancreaticoduodenectomy? Results of a prospective randomized placebo-controlled trial. Ann Surg 2000;232: 419–29.
- Lillemoe KD, Cameron JL, Kim MP, Campbell KA, Sauter PK, Coleman JA, . Does fibrin glue sealant decrease the rate of pancreatic fistula after pancreaticoduodenectomy? Results of a prospective randomized trial. J Gastrointest Surg. 2004;8:766–72.
- Yeo CJ, Barry MK, Sauter PK, Sostre S, Lillemoe KD, Pitt HA, . Erythromycin accelerates gastric emptying after pancreaticoduodenectomy. A prospective, randomized, placebo-controlled trial. Ann Surg. 1993;218:229–37.
- Hartel M, Wente MN, Hinz U, Kleeff J, Wagner M, Müller MW, . Effect of antecolic reconstruction on delayed gastric emptying after the pylorus-preserving Whipple procedure. Arch Surg. 2005;140:1094–9.
- Lillemoe KD, Kaushal S, Cameron JL, Sohn TA, Pitt HA, Yeo CJ. Distal pancreatectomy: Indications and outcomes in 235 patients. Ann Surg. 1999;229:693–8.
- Schnelldorfer T, Ware AL, Sarr MG, Smyrk TC, Zhang L, Qin R, . Long-term survival after pancreatoduodenectomy for pancreatic adenocarcinoma: Is cure possible? Ann Surg 2008;247:456–62.
- Bilimoria KY, Bentrem DJ, Ko CY, Tomlinson JS, Stewart AK, Winchester DP, . Multimodality therapy for pancreatic cancer in the U. S.: Utilization, outcomes, and the effect of hospital volume. Cancer. 2007;110:1227–34.
- Roldan GE, Gunderson LL, Nagorney DM, Martin JK, Ilstrup DM, Holbrook MA, . External beam versus intraoperative and external beam irradiation for locally advanced pancreatic cancer. Cancer. 1988;61:1110–6.
- Gastrointestinal Tumor Study Group. Further evidence of effective adjuvant combined radiation and chemotherapy following curative resection of pancreatic cancer. Cancer. 1987;59:2006–10.
- Neoptolemos JP, Dunn JA, Stocken DD, Almond J, Link K, Beger H, . Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: A randomised controlled trial. Lancet. 2001;358:1576–85.
- Klaassen DJ, MacIntyre JM, Catton GE, Engstrom PF, Moertel CG. Treatment of locally unresectable cancer of the stomach and pancreas: A randomized comparison of 5-fluorouracil alone with radiation plus concurrent and maintenance 5-fluorouracil–an Eastern Cooperative Oncology Group study. J Clin Oncol. 1985;3:373–8.
- Cohen SJ, Dobelbower R Jr, Lipsitz S, Catalano PJ, Sischy B, Smith TJ, . A randomized phase III study of radiotherapy alone or with 5-fluorouracil and mitomycin-C in patients with locally advanced adenocarcinoma of the pancreas: Eastern Cooperative Oncology Group study E8282. Int J Radiat Oncol Biol Phys. 2005;62:1345–50.
- Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, . Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: A randomized controlled trial. JAMA. 2007;297:267–77.
- Burris HA 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, . Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J Clin Oncol. 1997;15:2403–13.
- Picozzi VJ, Kozarek RA, Traverso LW. Interferon-based adjuvant chemoradiation therapy after pancreaticoduodenectomy for pancreatic adenocarcinoma Am J Surg 2003;185:476–80.
- Linehan DC, Tan MC, Strasberg SM, Drebin JA, Hawkins WG, Picus J, . Adjuvant interferon-based chemoradiation followed by gemcitabine for resected pancreatic adenocarcinoma: A single-institution phase II study. Ann Surg. 2008;248:145–51.
- Vauthey JN, Dixon E. AHPBA/SSO/SSAT Consensus Conference on Resectable and Borderline Resectable Pancreatic Cancer: Rationale and overview of the conference. Ann Surg Oncol. 2009;16:1725–6.
- Moutardier V, Magnin V, Turrini O, Viret F, Hennekinne-Mucci S, Gonçalves A, . Assessment of pathologic response after preoperative chemoradiotherapy and surgery in pancreatic adenocarcinoma. Int J Radiat Oncol Biol Phys. 2004;60:437–43.
- Evans DB, Rich TA, Byrd DR, Cleary KR, Connelly JH, Levin B, . Preoperative chemoradiation and pancreaticoduodenectomy for adenocarcinoma of the pancreas. Arch Surg. 1992;127:1335–9.
- Pisters PWT, Wolff RA, Crane CH, Evans DB. Combined-modality treatment for operable pancreatic adenocarcinoma. Oncology 2005; 19:393–404, 409.
- Abrams RA, Lowy AM, O’Reilly EM, Wolff RA, Picozzi VJ, Pisters PW. Combined modality treatment of resectable and borderline resectable pancreas cancer: Expert consensus statement. Ann Surg Oncol. 2009;16:1751–6.
- Cartwright TH, Cohn A, Varkey JA, Chen YM, Szatrowski TP, Cox JV, . Phase II study of oral capecitabine in patients with advanced or metastatic pancreatic cancer. J Clin Oncol. 2002;20:160–4.
- Andersen JR, Sorensen SM, Kruse A, Rokkjaer M, Matzen P. Randomised trial of endoscopic endoprosthesis versus operative bypass in malignant obstructive jaundice. Gut. 1989;30:1132–5.
- Smith AC, Dowsett JF, Russell RC, Hatfield AR, Cotton PB. Randomised trial of endoscopic stenting versus surgical bypass in malignant low bile duct obstruction. Lancet. 1994;344:1655–60.
- VanHeek NT, De Castro SM, vanEijck CH, vanGeenen RC, Hesselink EJ, Breslau PJ, . The need for a prophylactic gastrojejunostomy for unresectable periampullary cancer: A prospective randomized multicenter trial with special focus on assessment of quality of life. Ann Surg 2003;238: 894–902.
- Lillemoe KD, Cameron JL, Hardacre JM, Sohn TA, Sauter PK, Coleman J, . Is prophylactic gastrojejunostomy indicated for unresectable periampullary cancer? A prospective randomized trial. Ann Surg 1999;230: 322–8.
- Lillemoe KD, Cameron JL, Kaufman HS, Yeo CJ, Pitt HA, Sauter PK. Chemical splanchnicectomy in patients with unresectable pancreatic cancer. A prospective randomized trial. Ann Surg 1993;217: 447–55.
- Wong GY, Schroeder DR, Carns PE, Yeo CJ, Pitt HA, Sauter PK. Effect of neurolytic celiac plexus block on pain relief, quality of life, and survival in patients with unresectable pancreatic cancer: A randomized controlled trial. JAMA. 2004;291:1092–9.
- Levy MJ, Wiersema MJ. EUS-guided celiac plexus neurolysis and celiac plexus block. Gastrointest Endosc 2003;57: 923–30.