Abstract
Background. The benefit of adjuvant therapy for resected small bowel adenocarcinoma has not been proven. We undertook a retrospective analysis to evaluate the benefit of adjuvant therapy in a clearly defined patient population with curatively resected small bowel adenocarcinoma. Material and methods. We identified 54 patients with small bowel adenocarcinoma who underwent margin-negative surgical resection and were evaluated after surgery at the University of Texas, M. D. Anderson Cancer Center between 1990 and 2008. Disease-free survival (DFS) and overall survival (OS) were estimated. Results. Median age was 55 years and primary tumor site was duodenum in 67%, jejunum in 20%, and ileum in 13%. Thirty patients (56%) received adjuvant therapy consisting of systemic chemotherapy with or without radiation in 28 and radiation alone in two. Patients who received adjuvant therapy had significantly higher tumor stage and rate of lymph node involvement. Five-year DFS and OS did not differ between treatment groups. In multivariate analysis, the use of adjuvant therapy was associated with improved DFS (HR 0.27; 95% CI 0.07–0.98, P = 0.05) but not OS (HR 0.47; 95% CI 0.13–1.62, P = 0.23). In patients with a high risk of relapse (defined as a lymph node ratio ≥10%), adjuvant therapy appeared to improve OS, P = 0.04, but not DFS, P = 0.15. Discussion. The use of adjuvant therapy for curatively resected small bowel adenocarcinoma was associated with an improvement in DFS. This finding strongly supports further investigation of adjuvant chemotherapy in this tumor type.
Adenocarcinoma of the small intestine is a rare malignancy, with an estimated 1,992 new cases in the USA in the year 2008 [Citation1,Citation2]. The majority of patients present with locoregional disease, with stage II or III disease in 53% of patients [Citation3]. Outcomes for patients with stage II or stage III disease are poor, with data from the National Cancer Database demonstrating 5-year disease-specific survivals of 48% and 35%, respectively [Citation3]. Outcomes from large single institution centers are similar, with reported 5-year overall survivals (OS) for patients with resected small bowel adenocarcinoma of 27 to 52% [Citation4–7].
The rationale to investigate adjuvant chemotherapy in small bowel adenocarcinoma has been based upon both the known pattern of failure following surgical resection and the reported activity of systemic chemotherapy in patients with advanced disease. In patients with advanced disease, modern chemotherapy combinations of 5-fluorouracil and a platinum agent have demonstrated significant activity, with response rates ranging from 29 to 50% [Citation8–10]. In retrospective series that have evaluated the pattern of initial disease recurrence following surgical resection, distant relapse occurred in 81 to 100% of cases, while local relapse occurred in 0 to 29% of cases [Citation4,Citation5,Citation7,Citation11]. In the subset of patients with adenocarcinoma of the duodenum, the rate of local relapse is higher, but systemic relapse still predominates [Citation4,Citation5,Citation7,Citation11–14].
A number of single institution retrospective studies have evaluated the role of adjuvant chemotherapy for small bowel adenocarcinoma, but none of these studies have shown a statistically significant benefit favoring its use [Citation4,Citation5,Citation7,Citation11]. Separate retrospective studies have evaluated the role of adjuvant chemoradiation following resection of adenocarcinoma of the duodenum, but none have demonstrated a clear benefit for adjuvant treatment [Citation12–15]. The inability to control for the various prognostic factors influencing the original decision to administer adjuvant therapy has been a major limitation of these studies, as patients who receive adjuvant therapy tend to be those at higher risk for disease recurrence.
Despite the lack of evidence supporting the delivery of adjuvant chemotherapy for small bowel adenocarcinoma, an analysis of the National Cancer Database has shown an increase in use from 8% in 1985 to 24% in 2005 [Citation1]. This likely reflects an extrapolation of the results supporting adjuvant chemotherapy in the treatment of colorectal cancer [Citation16]. However, the validity of such an extrapolation is not known.
We undertook this retrospective analysis to evaluate the benefit of adjuvant therapy in a clearly defined patient population having undergone a curative resection of small bowel adenocarcinoma. Only patients evaluated at The University of Texas M. D. Anderson Cancer Center (UTMDACC) after a margin-negative surgical resection were eligible.
Material and methods
A total of 202 patients with small bowel adenocarcinoma between 1990 and 2006 were identified in the UTMDACC Tumor Registry. All patients were required to have tumor histology that was reviewed at our center and consistent with small bowel adenocarcinoma. Peri-operative radiographic imaging demonstrating no evidence of metastatic disease within the month prior to surgery or within three months following surgery was required. We limited the analysis to patients with non-metastatic disease who underwent a margin-negative resection and had a postoperative outpatient appointment at UTMDACC within three months of surgery. Patients who had received adjuvant therapy prior to an evaluation at UTMDACC were excluded as were patients who received neoadjuvant therapy (chemotherapy or radiation therapy). Reasons for exclusion included stage IV disease in 91 patients, recurrent disease following prior curative surgical resection in 20 patients, palliative surgery in 19 patients, positive resection margins in nine patients, lack of surgical resection in six patients, use of neoadjuvant therapy in five patients, no outpatient follow-up appointment within three months of surgery in five patients, and no pathological confirmation in two patients. Patient records were retrospectively reviewed, and information regarding demographic data, tumor characteristics, treatment, and survival was extracted. This study was performed in accordance with the institutional review board guidelines.
Statistical analysis
Descriptive statistics were used to describe the basic features of the data in the study. Wilcoxon rank sum test was used to assess the difference in continuous variables between the treatment groups. Fisher’s exact test was used to assess the association between categorical variables. The Kaplan-Meier product-limit method was used to estimate DFS/OS and log-rank test was used to compare DFS/OS between treatment groups. Univariate and multivariate Cox proportional hazard models were fit for DFS/OS. The covariates examined including age, tumor differentiation, lymph node involvement, tumor stage, primary site, and use of adjuvant therapy. A lymph node ratio of ≥10% has previously been shown to be a strong prognostic variable in small bowel adenocarcinoma and was therefore included as a covariate [Citation17]. In the multivariate Cox proportional hazards model, step-wise model selection method was performed to obtain a best subset of variables in the final model. P-values were derived from two-sided tests, and the statistical analyses were performed using SAS 9.1 (SAS Institute Inc., 2002–2003) and Splus 7.0 (MathSoft, Inc., 2005).
Results
Patients and treatment
Baseline characteristics of patients treated with and without adjuvant therapy are shown in . Patients who received adjuvant therapy were more likely to be younger (p = 0.03), have a higher tumor stage (p ≤ 0.01) and have involved lymph nodes (p = 0.01). Of the patients who had lymph node involvement the lymph node ratio (number of positive to total lymphnodes) was higher in patients who received adjuvant therapy, p = 0.03.
Table I. Patient and treatment characteristics by treatment group.
Of the 30 patients who received adjuvant therapy, 28 received chemotherapy as a component of their adjuvant treatment (). In particular, adjuvant therapy consisted of systemic chemotherapy alone in 18 patients, systemic chemotherapy plus chemoradiation in seven, chemoradiation in three, and radiation therapy alone in two. Four of the five patients who received local therapy alone had duodenal adenocarcinoma. Two of these patients had node negative disease and one patient had a close duodenal margin. The one patient with adenocarcinoma of the jejunum who received local therapy alone had a T4N0 tumor with direct extension into the kidney. All patients who received chemotherapy had either intravenous 5-fluorouracil or capecitabine as a component of their chemotherapy regiment.
Table II. Adjuvant treatment.
Survival analysis
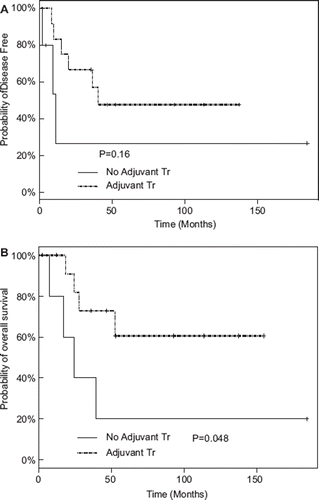
At a median follow-up of 4.7 years, 14 patients (26%) have relapsed and 12 patients (22%) have died. When stratified by stage, the 5-year OS for patients with stage I, II, and III disease were 100% (95% CI: 100–100%), 87% (95% CI: 29–98%), and 59% (95% CI: 25–81%). Both DFS and OS were not statistically different between patients with and without adjuvant therapy (). In univariate analysis, adjuvant therapy did not statistically improve the outcomes ().
Table III. Univariate Cox proportional hazards model in estimating the association between patient characteristics and survival.
Figure 1. Kaplan-Meier plots stratified by use of adjuvant chemotherapy for disease-free survival (A) and overall survival (B).
Multivariate analysis was performed to evaluate the effect of adjuvant therapy while adjusting for other covariates (). For both OS and DFS, a lymph node ratio of ≥10% and poorly differentiated histology were associated with worse outcomes. For DFS, adjuvant therapy was associated with an improved outcome, with a hazard ratio of 0.27 (95% confidence interval [CI] 0.07–0.98, p = 0.05). For OS, no benefit from adjuvant therapy was seen, with a hazard ratio of 0.47 (95% CI 0.13–1.62, p = 0.23).
Table IV. Multivariate Cox proportional hazard ratios for DFS/OS.
To better evaluate the role of systemic chemotherapy as adjuvant therapy, two separate multivariate analyses were conducted. In the first analysis we compared patients who had received systemic adjuvant chemotherapy with or without additional radiation (n = 25) to patients who received no adjuvant therapy. Adjuvant therapy improved DFS (HR 0.23, 95% CI: 0.06–0.89, p = 0.03) but not OS (HR 0.48, 95% CI: 0.13–1.74, p = 0.26). In the second analysis patients treated with adjuvant systemic chemotherapy alone (n = 18) were compared to patients who received no adjuvant therapy. Though the hazard ratios were similar in this analysis, adjuvant chemotherapy was not a statistically significant factor for either DFS (HR 0.33, 95% CI: 0.08–1.27, p = 0.11) or OS (HR 0.53, 95% 0.14–2.06, p = 0.36).
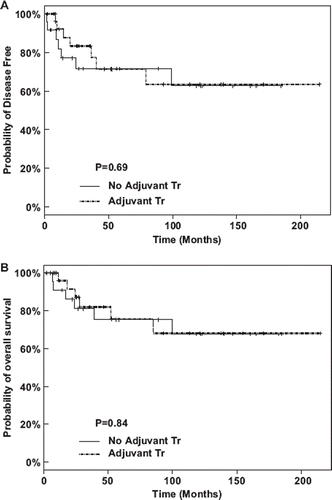
In the subgroup of patients with high risk disease, as defined by a lymph node ratio ≥10%, adjuvant therapy was associated with an improvement in OS (median OS: >;150 months vs. 24 months, p = 0.04), but not DFS (median DFS: 40 months vs. 11 months, p = 0.15), .
Discussion
Although no statistical difference in DFS or OS was observed between patients who did and did not receive adjuvant therapy, patients receiving adjuvant therapy had significantly more adverse prognostic factors. After adjusting for these factors in the multivariate model, adjuvant therapy was associated with an improvement in DFS. The results of this study, though limited by the small sample size, strongly support the further investigation of adjuvant therapy in patients with small bowel adenocarcinoma.
Due to the rarity of small bowel adenocarcinoma, no prospective study has evaluated the role of adjuvant therapy in this disease. Retrospective studies from a number of single institutions have evaluated the role of adjuvant therapy, although the majority of these studies have included all stages of disease and have not provided a subgroup analysis of patients with margin-negative resections [Citation4,Citation5,Citation8]. In a retrospective series of 64 patients from Roswell Park Cancer Institute, 30 patients underwent margin-negative resections and 11 of these patients received adjuvant chemotherapy [Citation4]. Median OS for those patients who did and did not receive adjuvant chemotherapy were 56 and 41 months, respectively, though no statistical comparison was conducted. A second study from the Princess Margaret Hospital reported on 60 patients who underwent curative-intent surgery with unknown margin status [Citation8]. Fifteen patients (25%) received adjuvant chemotherapy, with a median OS of 22 months compared with 28 months for the non-adjuvant treatment group. However, patients receiving adjuvant therapy were more likely to have lymph node involvement and poor histological differentiation compared with patients who did not receive adjuvant treatment. A preliminary report from the Mayo Clinic also found no benefit with adjuvant therapy, primarily chemoradiation, in patients who underwent complete resection, even after adjusting for various prognostic factors, such as lymph node status, grade, and age [Citation18].
A large retrospective series from the M. D. Anderson Cancer Center reported upon 120 patients with small bowel adenocarcinoma who underwent surgical resection for non-metastatic disease [Citation5]. Fifty-eight patients received adjuvant chemotherapy with no benefit in OS, p = 0.49. Unfortunately, no information regarding the patients who did or did not receive adjuvant therapy was provided, and no multivariate analysis of these subgroups was conducted. In addition, this study was likely affected by referral bias, as it included patients who had undergone prior surgical resection for localized disease and subsequently presented to M. D. Anderson with recurrent disease.
Separate studies have evaluated the role of adjuvant therapy in patients with adenocarcinoma of the duodenum. A single prospective study, conducted by the European Organization for Research and Treatment of Cancer (EORTC), evaluated the role of adjuvant chemoradiation with 5-fluorouracil compared with observation in 93 patients with resected non-pancreatic periampullary cancer [Citation19]. No difference in survival was seen between the two arms, p = 0.74. A retrospective study of patients with duodenal adenocarcinoma from Duke University Medical Center compared 16 patients treated with surgery alone and 16 patients treated with either preoperative or postoperative chemoradiation [Citation20]. Five-year OS did not differ between patients who received and did not receive adjuvant chemoradiation, 57% compared with 44%, respectively, p = 0.42. In the subgroup of patients who underwent a margin-negative resection, patients who received chemoradiation appeared to have an improvement in 5-year OS, 83% compared with 53%, p = 0.07.
The study reported here is unique in comparison to these prior studies. First, this study selected only patients who had undergone a margin-negative resection. Second, only patients who had an outpatient evaluation within three months of surgical resection and prior to the initiation of adjuvant therapy were included. Third, a thorough comparison of prognostic factors between patients who did and did not receive adjuvant therapy was conducted. Despite the inclusion of a selected subset of patients with small bowel adenocarcinoma, the prognostic factors determined from this study are in agreement with the known poor prognostic factors in this tumor type, which have included T4 tumor stage, poor histological differentiation, positive resection margins, lymphovascular invasion and lymph node involvement [Citation4,Citation5,Citation7,Citation11,Citation21–24].
There are limitations to this data. This was a retrospective study with a potential for both selection and interpretation bias. The decision to administer adjuvant chemotherapy was not standardized and even though an extensive comparison of known prognostic factors was conducted, it is likely that additional factors affected decision-making. Furthermore, adjuvant therapy was not homogenous, although the vast majority of patients received fluoropyrimidine chemotherapy. Despite representing a rather large cohort of small bowel adenocarcinoma patients, interpretation of variables within this data set is limited by small sample size and interpretation of the results of this retrospective study must be done cautiously.
Given the rarity of small bowel adenocarcinoma, it is extremely unlikely that a prospective study will be conducted in this tumor type to address the role of adjuvant therapy. In this context, this report adds to the limited data that practicing clinicians can use to determine treatment options for patients with resected small bowel adenocarcinoma.
Acknowledgements
Financial support was provided by the Kavanagh Foundation.
Declaration of interest: The authors report no coflict of interest. The author alone are responsible for the content and writing of the paper.
References
- Bilimoria KY, Bentrem DJ, Wayne JD, Ko CY, Bennett CL, Talamonti MS. Small bowel cancer in the United States: Changes in epidemiology, treatment, and survival over the last 20 years. Ann Surg 2009;249:63–71.
- Jemal A, Siegel R, Ward E, . Cancer statistics, 2008. CA Cancer J Clin 2008;58:71–96.
- Howe JR, Karnell LH, Menck HR, Scott-Conner C. The American College of Surgeons Commission on Cancer and the American Cancer Society. Adenocarcinoma of the small bowel: Review of the National Cancer Data Base, 1985–1995. Cancer 1999;86:2693–706.
- Agrawal S, McCarron EC, Gibbs JF, Nava HR, Wilding GE, Rajput A. Surgical management and outcome in primary adenocarcinoma of the small bowel. Ann Surg Oncol 2007;14:2263–9.
- Dabaja BS, Suki D, Pro B, Bonnen M, Ajani J. Adenocarcinoma of the small bowel: Presentation, prognostic factors, and outcome of 217 patients. Cancer 2004;101:518–26.
- Ito H, Perez A, Brooks DC, . Surgical treatment of small bowel cancer: A 20-year single institution experience. J Gastrointest Surg 2003;7:925–30.
- Wu TJ, Yeh CN, Chao TC, Jan YY, Chen MF. Prognostic factors of primary small bowel adenocarcinoma: Univariate and multivariate analysis. World J Surg 2006;30:391–8; Discussion 399.
- Fishman PN, Pond GR, Moore MJ, . Natural history and chemotherapy effectiveness for advanced adenocarcinoma of the small bowel: A retrospective review of 113 cases. Am J Clin Oncol 2006;29:225–31.
- Overman MJ, Kopetz S, Wen S, . Chemotherapy with 5-fluorouracil and a platinum compound improves outcomes in metastatic small bowel adenocarcinoma. Cancer 2008;113: 2038–45.
- Overman MJ, Varadhachary GR, Kopetz S, . Phase II study of capecitabine and oxaliplatin for advanced adenocarcinoma of the small bowel and ampulla of vater. J Clin Oncol 2009.
- Bauer RL, Palmer ML, Bauer AM, Nava HR, Douglass HO, Jr. Adenocarcinoma of the small intestine: 21-year review of diagnosis, treatment, and prognosis. Ann Surg Oncol 1994;1:183–8.
- Bakaeen FG, Murr MM, Sarr MG, . What prognostic factors are important in duodenal adenocarcinoma? Arch Surg 2000;135:635–41.
- Barnes G, Jr., Romero L, Hess KR, Curley SA. Primary adenocarcinoma of the duodenum: Management and survival in 67 patients. Ann Surg Oncol 1994;1:73–8.
- Swartz MJ, Hughes MA, Frassica DA, . Adjuvant concurrent chemoradiation for node-positive adenocarcinoma of the duodenum. Arch Surg 2007;142:285–8.
- Kelsey CR, Nelson JW, Willett CG, . Duodenal adenocarcinoma: Patterns of failure after resection and the role of chemoradiotherapy. Int J Radiat Oncol Biol Phys 2007;69:1436–41.
- Andre T, Boni C, Mounedji-Boudiaf L, . Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 2004;350:2343–51.
- Overman MJ, Hu C, Wolff RA, Chang GJ. Impact of lymph node evaluation on survival for small bowel adenocaricnoma: Analysis of the Surveillance, Epidemiology and End Results (SEER) database. Presented at the 2009 ASCO Annual Meeting, Orlando Florida, #4596.
- Halfdanarson T, Quevedo F, McWilliams RR. Small bowel adenocarcinoma: A review of 491 cases (abstract). J Clin Oncol 2006;24:209s.
- Klinkenbijl JH, Jeekel J, Sahmoud T, . Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: Phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg 1999;230:776–82.
- Kelsey CR. Adenocarcinoma of the duodenum: The role of radiation therapy. Paper presented at: Gastrointestinal Cancers Symposium abstract 185, 2007.
- Abrahams NA, Halverson A, Fazio VW, Rybicki LA, Goldblum JR. Adenocarcinoma of the small bowel: A study of 37 cases with emphasis on histologic prognostic factors. Dis Colon Rectum 2002;45:1496–502.
- Brucher BL, Stein HJ, Roder JD, . New aspects of prognostic factors in adenocarcinomas of the small bowel. Hepatogastroenterology 2001;48:727–32.
- Ouriel K, Adams JT. Adenocarcinoma of the small intestine. Am J Surg 1984;147:66–71.
- Talamonti MS, Goetz LH, Rao S, Joehl RJ. Primary cancers of the small bowel: Analysis of prognostic factors and results of surgical management. Arch Surg 2002;137: 564–70.