Abstract
Background. A Joint Expert Panel recently published guidelines for adjuvant cisplatin-based chemotherapy, recommending routine use in patients with completely resected stage II (T1-2N1 and T3N0) non-small-cell lung cancer (NSCLC). However, these two tumor subgroups should be considered as different entities. While the efficacy of adjuvant chemotherapy has been established in patients with T1-2N1 NSCLC, its benefit in patients with T3N0 tumor remains questionable. Material and methods. We performed an extensive review of the literature using the Joint Expert Panel guidelines as a start point. Altogether, we identified 76 potentially relevant articles. Basing on inclusion and exclusion criteria, 23 of the 76 articles were eventually included in this review. Results. After careful evaluation of the selected articles, we found no information on the effect of adjuvant chemotherapy in patients with T3N0 NSCLC. Discussion. In the absence of evidence-based data, we recommend that the lack of information on the efficacy of adjuvant chemotherapy for T3N0 tumors be discussed with patients and propose chemotherapy as an individual option. While the efficacy of adjuvant chemotherapy will be difficult to assess prospectively through a large randomized clinical trial, a pooled-analysis of the existing data would quickly and with a limited effort provide a preliminary answer.
Introduction
The role of adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer (NSCLC) has been evaluated in many randomized clinical trials (RCT) and meta-analyses. In August 2006, the Cancer-Care-Ontario-Program in Evidence-Based Care and the American-Society of Clinical-Oncology convened a Joint Expert Panel to review the evidence and draft recommendations for these therapies, and concluded that adjuvant cisplatin-based chemotherapy is recommended for routine use in patients with stages IIA, IIB according to the 6th edition of the TNM Classification of Malignant Tumors, that means in patients with T1-2N1 or T3N0 NSCLC [Citation1].
In the 6th edition of the pTNM, patients with pT3N0 and pT1-2N1 NSCLC were gathered together as stage II, either IIA (pT1N1) or IIB (pT2N1 or pT3N0), because of their similar prognosis. However it should be kept in mind that a similar prognosis in different subgroups does not mean similar efficacy of adjuvant treatments. In fact there are grounds for considering T3N0 and T1-2N1 NSCLC as the same disease, but with differences in their biology. Indeed the different anatomic extent of these tumors might be responsible and/or epiphenomenon for the different biological behavior and response to adjuvant chemotherapy. T3N0 NSCLC are tumors that, despite local progression (i.e. chest wall and/or diaphragm and/or mediastinal pleura and/or parietal pericardium invasion), does not spread to lymph nodes unlike T1-2N1 disease which even in the absence of invasion of surrounding tissues has spread to at least one regional (i.e., hilar and/or peribronchial) lymph node. Also extensive local treatment and/or major surgical procedures often required in T3N0 disease (i.e.: tumor closer than 2 cm from carina, in-toto obstructive pneumonitis/atelectasia), can lead to different reasons and pattern of failures compared with T1-2N1 tumors.
Differences in the behavior of T3N0 and T1-2N1 NSCLC is also suggested by different biological characteristics: a retrospective analysis of 435 consecutive patients with stage II NSCLC treated with upfront surgery in our institute (unpublished data) indicated that vascular invasion was more often present in T1-2N1 than in T3N0 tumors (37% vs. 14%; p=0.001) and was observed both in adenocarcinomas (44% vs. 22%; p=0.05) and in squamous-cell carcinomas (29% vs. 0%, p=0.002).
Based on these considerations and on the fact that efficacy of adjuvant chemotherapy usually varies according to lymph nodal status [Citation2], the efficacy of adjuvant chemotherapy should be assessed separately in the T1-2N1 and T3N0 sub-groups. A pooled-analysis of the largest RCT [Citation3] has already demonstrated an efficacy of adjuvant chemotherapy in patients with T1-2N1 NSCLC, which corresponded to stage II of the 1986 classification of the American Joint Committee on Cancer [Citation4] used in these RCT. Although adjuvant chemotherapy was efficacious in stage II NSCLC (1986 classification), we are unsure that the potential benefit on overall and disease-free survival to the T3N0 NSCLC subgroup of patients, which represent about 20% of NSCLC stage II. Furthermore the proposed modifications for the 7th edition of the TNM [Citation5] suggest that patients with T3 (invasion) No tumors will be combined with T2 (>7 cm) N0 patients and classified as pathologic stage IIB, whereas T2N0 patients are now all included in stage IB (independent of size), for whom adjuvant chemotherapy is currently not recommended [Citation1,Citation6].
To assess if the available data support the current recommendation for routine use of adjuvant chemotherapy in patients with T3N0 NSCLC, we performed a literature search starting with the Cancer Care Ontario and American Society of Clinical Oncology guidelines [Citation1].
Literature review
The Joint Expert Panel guidelines collected evidence on the efficacy of platinum-based chemotherapy in resectable NSCLC from eight previously published meta-analyses [Citation7–16] and 16 RCT [Citation2,Citation6,Citation17–30]. Moreover, the primary source of evidence for these practice guidelines came from three systematic reviews [Citation31–33]. Since there was no separate data for treated and non-treated patients with T3N0 NSCLC in either the meta-analyses or the reviews, we extended the literature search to all the studies cited in the references of these reports. Altogether, we identified 76 potentially relevant articles. We considered eligible any RCT comparing adjuvant chemotherapy versus no chemotherapy (or adjuvant chemotherapy plus radiotherapy versus adjuvant radiotherapy alone) in patients with completely resected T3N0 NSCLC. We also included trials using non cisplatin-based regimens in this review. When trials included patients with both resected and non-resected NSCLC, only the results relevant to patients with resected tumor were considered. Trials evaluating pre- or peri-operative chemotherapy or evaluating efficacy of chemoimmunotherapy were excluded. Based on these inclusion criteria, we selected 23 of the 76 articles examined. After an extensive review of the 23 independent RCT [Citation2,Citation17,Citation18,Citation20–22,Citation25–27,Citation34–47] we found no information on the effect of adjuvant chemotherapy in the subset of patients with completely resected T3N0 NSCLC.
Proposals and perspectives
The remaining options to assess whether adjuvant chemotherapy is effectively useful in T3N0 NSCLC patients are either to perform a pooled-analysis of individual data from the existing published RCT or to start an ad-hoc prospective multicentric RCT.
To evaluate the feasibility of a pooled-analysis of existing data, we retrieved (or estimated) data on the patients with completely resected T3N0 NSCLC enrolled in the 23 published RCT. Four papers [Citation18,Citation20,Citation27,Citation41] reported the exact number of patients with T3N0 NSCLC both in the adjuvant chemotherapy and in the control group, while one [Citation42] reported survival only for treated patients. For the other studies, we estimated the number of patients with T3N0 tumors based on the stage distribution observed in the International Adjuvant Lung Trial (IALT [Citation27]). As a result, we estimated the number of treated T3N0 NSCLC patients to be 369 and 340 the non-treated patients.
Statistical considerations
We estimated the statistical power of an eventual pooled-analysis of the previous reports (based on 369 treated and 340 non-treated T3N0 NSCLC patients) using an equivalence test of proportion with a 5% type I one-sided error rate, assuming that the five year overall survival of untreated T3N0 NSCLC patients to be 39% [Citation1].
If we consider equivalent an absolute benefit of adjuvant chemotherapy <5% (i.e. the percentage considered significant in previous meta- and pooled-analyses [Citation48]) the power will be 39%, but it will reach 80% if we consider equivalent an absolute benefit of adjuvant chemotherapy <9% ().
Figure 1. Variation of statistical power and sample size (N) considering equivalence in survival of treated and untreated patients, for different maximum allowable difference (Delta) in survival at five years. Horizontal dashed line represents 80% statistical power, vertical dashed line corresponds to the estimated number of treated patients (N=369) enrolled in existing studies.
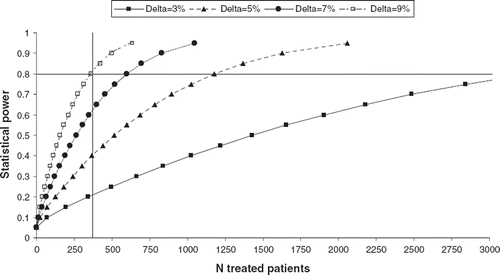
If we intend to initiate an ad-hoc prospective multicentric RCT, specifically designed to assess the inefficacy of adjuvant chemotherapy in T3N0 NSCLC patients, a sample size of 1 176 treated and an equal number of not treated patients will be required to obtain a statistical power of 80% in order to identify no significant difference in survival in the two groups (absolute improvement of less than 5% at five years in patients receiving adjuvant chemotherapy). A similar number of patients will be required to detect a significant improvement in survival of at least 5% in T3N0 patients.
Variations of the statistical power and sample size for different values of the maximum allowable improvement in survival at five years in patients receiving adjuvant chemotherapy are shown in .
Discussion
Our literature review provided no evidence for the efficacy of adjuvant chemotherapy in patients with stage II T3N0 NSCLC.
In contrast to the Joint Expert Panel recommendations, we believe that without proofs of efficacy, patients should not be systematically exposed to the risks and adverse effects of adjuvant chemotherapy, including relevant affects on the patient’s quality of life. In the LACE pooled-analysis [Citation3] the rate of overall grade 3 or 4 toxicity was 66%; overall grade 4 toxicity alone was 32%. Toxicity-related mortality in the three largest studies [Citation2,Citation3,Citation29] ranged from 0.8 to 2%.
We recommend that physicians offering the option of adjuvant chemotherapy to patients should explain that its efficacy is still to be proven. A case-by-case decision should be shared with the patient based on the evaluation of other prognostic factors, such as grade, vascular invasion and PET scan and the patient’s attitude.
There is still an urgent need to conclusively assess the efficacy of adjuvant chemotherapy in resected T3N0 NSCLC. We propose a retrospective approach, based on a pooled-analysis of available data, as a randomized prospective approach would require enormous effort, a lengthy recruitment period and far too long before the first meaningful results are attained.
The advantage of a pooled-analysis of existing data is that patients have already been enrolled and survival data are available, so an initial answer to our question could be provided in a short-time period. While we estimated that this pooled-analysis could have enough power to detect a large difference in survival (>9%), the estimated limited number of available patients with T3N0 NSCLC will confer a low statistical power (39%) to assess any difference in survival (less than 5% at five years).
Conclusion
After careful evaluation, we conclude that the Joint Expert Panel Guidelines for patients with stage II NSCLC should not be generalized to patients with completely resected T3N0 tumors. In the absence of evidence-based data and taking into account the cost and potential toxicity of adjuvant chemotherapy, we recommend discussion with patients on the lack of information on the efficacy of adjuvant chemotherapy for T3N0 tumors, and propose it as an individual option. While the efficacy of such adjuvant treatment will be difficult to assess prospectively through a RCT, a pooled-analysis of the existing data would quickly and with limited effort provide a preliminary answer.
Acknowledgments
The authors would like to thank William Russell-Edu for his valuable library assistance and Linda Cairns for grammatical correction of the paper.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Pisters KM, Evans WK, Azzoli CG, Kris MG, Smith CA, Desch CE, . Cancer Care Ontario and American Society of Clinical Oncology adjuvant chemotherapy and adjuvant radiation therapy for stages I-IIIA resectable non small-cell lung cancer guideline. J Clin Oncol 2007;25:5506–18.
- Douillard JY, Rosell R, De Lena M, Carpagnano F, Ramlau R, Grodzki T, . Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): A randomised controlled trial. Lancet Oncol 2006;7:719–27.
- Pignon JP, Tribodet H, Scagliotti GV, Douillard JY, Shepherd FA, Stephens RJ, . Lung adjuvant cisplatin evaluation: A pooled analysis by the LACE Collaborative Group. J Clin Oncol 2008;26:3552–9.
- Mountain CF. A new international staging system for lung cancer. Chest 1986;89:S225–S233.
- Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, . The IASLC Lung Cancer Staging Project: Proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007; 2:706–14.
- Strauss GM, Herndon J E, Maddaus MA. Adjuvant chemotherapy in stage IB non small cell lung cancer (NSCLC): Update of Cancer and Leukemia Group B (CALGB) protocol 19633. J Clin Oncol 2008;24:S36565(Suppl; abstr 7007).
- Hamada C, Tanaka F, Ohta M, Fujimura S, Kodama K, Imaizumi M, . Meta-analysis of postoperative adjuvant chemotherapy with tegafur-uracil in non-small-cell lung cancer. J Clin Oncol 2005;23:4999–5006.
- Non-Small Cell Lung Cancer Collaborative Group. Chemotherapy in non-small cell lung cancer: A meta-analysis using updated data on individual patients from 52 randomised clinical trials. BMJ 1995;311:899–909.
- George S, Schell MJ, Detterbeck FC, Socinski MA. Adjuvant chemotherapy for resected non-small cell carcinoma of the lung: Why we still don’t know. Oncologist 1998;3:35–44.
- Bria E, Felici A, Carlini P, Ferretti G, Nistico C, Cuppone F, . Adjuvant chemotherapy for non-small cell lung cancer: Pooled analysis of 3443 patients. Ann Oncol 2004; 15:A646 (Suppl 3).
- Hotta K, Matsuo K, Ueoka H, Kiura K, Tabata M, Tanimoto M. Role of adjuvant chemotherapy in patients with resected non-small-cell lung cancer: Reappraisal with a meta-analysis of randomized controlled trials. J Clin Oncol 2004;22:3860–7.
- Sedrakyan A, Van Der MJ, O’Byrne K, Prendiville J, Hill J, Treasure T. Postoperative chemotherapy for non-small cell lung cancer: A systematic review and meta-analysis. J Thorac Cardiovasc Surg 2004;128:414–9.
- Berghmans T, Paesmans M, Meert AP, Mascaux C, Lothaire P, Lafitte JJ, . Survival improvement in resectable non-small cell lung cancer with (neo)adjuvant chemotherapy: Results of a meta-analysis of the literature. Lung Cancer 2005;49:13–23.
- Bria E, Gralla R, Raftopoulos H, Ferretti G, Felici A, Nistico C, . Does adjuvant chemotherapy improve survival in non small cell lung cancer (NSCLC)? A pooled-analysis of 6494 patients in 12 studies, examining survival and magnitude of benefit. J Clin Oncol 2005;23(Suppl):655s.
- Pignon JP, Tribodet H, Scagliotti GV, Douillard JY, Shepherd FA, Stephens RJ, . Lung Adjuvant Cisplatin Evaluation (LACE): A pooled analysis of five randomized clinical trials including 4,584 patients. J Clin Oncol 2006;24(Suppl):S366.
- Stewart LA, Burdett S, Tierney JF, Pignon J. Surgery and adjuvant chemotherapy (CT) compared to surgery alone in non-small cell lung cancer (NSCLC): A meta-analysis using individual patient data (IPD) from randomized clinical trials (RCT). J Clin Oncol 2007;25(Suppl):S397.
- Ichinose Y, Hara N, Ohta M, Motohiro A, Kuda T, Aso H. Postoperative adjuvant chemotherapy in non-small cell lung cancer: Prognostic value of DNA ploidy and post-recurrent survival. J Surg Oncol 1991;46:15–20.
- Niiranen A, Niitamo-Korhonen S, Kouri M, Assendelft A, Mattson K, Pyrhonen S. Adjuvant chemotherapy after radical surgery for non-small-cell lung cancer: A randomized study. J Clin Oncol 1992;10:1927–32.
- Feld R, Rubinstein L, Thomas PA. Adjuvant chemotherapy with cyclophosphamide, doxorubicin, and cisplatin in patients with completely resected stage I non-small-cell lung cancer. The Lung Cancer Study Group. J Natl Cancer Inst 1993; 85:299–306.
- Ohta M, Tsuchiya R, Shimoyama M, Sawamura K, Mori T, Miyazawa N, . Adjuvant chemotherapy for completely resected stage III non-small-cell lung cancer. Results of a randomized prospective study. The Japan Clinical Oncology Group. J Thorac Cardiovasc Surg 1993;106:703–8.
- Dautzenberg B, Chastang C, Arriagada R, Le Chevalier T, Belpomme D, Hurdebourcq M, . Adjuvant radiotherapy versus combined sequential chemotherapy followed by radiotherapy in the treatment of resected nonsmall cell lung carcinoma. A randomized trial of 267 patients. GETCB (Groupe d’Etude et de Traitement des Cancers Bronchiques). Cancer 1995;76:779–86.
- Lee CG, Kim JH, Chung KY, Lee DY, Kim KD, Lee WY . A prospective randomized study of postoperative adjuvant chemo-radiotherapy (CT+RT) vs radiotherapy (RT) alone in resected stage II and IIIA non-small call lung cancer (NSCLC). Int J Radiat Oncol Biol Phys 1995;32:280(Suppl 1).
- Keller SM, Adak S, Wagner H, Herskovic A, Komaki R, Brooks BJ, . A randomized trial of postoperative adjuvant therapy in patients with completely resected stage II or IIIA non-small-cell lung cancer. Eastern Cooperative Oncology Group. N Engl J Med 2000;343:1217–22.
- Mineo TC, Ambrogi V, Paci M, Iavicoli N, Pompeo E, Nofroni I. Transxiphoid bilateral palpation in video-assisted thoracoscopic lung metastasectomy. Arch Surg 2001;136: 783–8.
- Lee G-W, Kim S-W, Suh C, Kim WS, Kim DK, Park SI, . Prognostic significance of lympho/vascular tumor emboli at adjuvant chemotherapy setting in the patients with stage II, III non-small cell lung cancer after curative resection and postoperative thoracic radiotherapy. Proc Am Soc Clin Oncol 2003;21:649.
- Scagliotti GV, Fossati R, Torri V, Crino L, Giaccone G, Silvano G, . Randomized study of adjuvant chemotherapy for completely resected stage I, II, or IIIA non-small-cell Lung cancer. J Natl Cancer Inst 2003;95:1453–61.
- Arriagada R, Bergman B, Dunant A, Le Chevalier T, Pignon JP, Vansteenkiste J. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med 2004;350:351–60.
- Tada H, Tsuchiya R, Ichinose Y, Koike T, Nishizawa N, Nagai K, . A randomized trial comparing adjuvant chemotherapy versus surgery alone for completely resected pN2 non-small cell lung cancer (JCOG9304). Lung Cancer 2004;43:167–73.
- Winton T, Livingston R, Johnson D, Rigas J, Johnston M, Butts C, . Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med 2005; 352:2589–97.
- Park JH, Lee CT, Lee HW, Baek HJ, Zo JI, Shim YM. Postoperative adjuvant chemotherapy for stage I non-small cell lung cancer. Eur J Cardiothorac Surg 2005;27:1086–91.
- Logan DM, Lochrin CA, Darling G, Eady A, Newman TE, Evans WK. Adjuvant radiotherapy and chemotherapy for stage II or IIIA non-small-cell lung cancer after complete resection. Provincial Lung Cancer Disease Site Group. Cancer Prev Control 1997;1:366–78.
- Alam N, Darling G, Evans WK, Mackay JA, Shepherd FA. Adjuvant chemotherapy for completely resected non-small cell lung cancer: A systematic review. Crit Rev Oncol Hematol 2006;58:146–55.
- Alam N, Darling G, Shepherd FA, Mackay JA, Evans WK. Postoperative chemotherapy in nonsmall cell lung cancer: A systematic review. Ann Thorac Surg 2006;81:1926–36.
- Dillman RO, Seagren SL, Propert KJ, Guerra J, Eaton WL, Perry MC, . A randomized trial of induction chemotherapy plus high-dose radiation versus radiation alone in stage III non-small-cell lung cancer. N Engl J Med 1990;323:940–5.
- Osaka Lung Cancer Study Group. Postoperative adjuvant chemotherapy in non-small cell lung cancer: Results of a prospective randomized study. Jpn J Lung Cancer 1992; 32:11–22.
- Imaizumi, M. Postoperative adjuvant cisplatin, vindesine, plus uracil-tegafur chemotherapy increased survival of patients with completely resected p-stage I non-small cell lung cancer. Lung Cancer 1995;49:85–94.
- Wada H, Hitomi S, Teramatsu T. Adjuvant chemotherapy after complete resection in non-small-cell lung cancer. West Japan Study Group for Lung Cancer Surgery. J Clin Oncol 1996;14:1048–54.
- Xu G, Rong T, Lin P. Adjuvant chemotherapy following radical surgery for non-small-cell lung cancer: A randomized study on 70 patients. Chin Med J (Engl) 2000;113:617–20.
- Waller D, Peake MD, Stephens RJ, Gower NH, Milroy R, Parmar MK, . Chemotherapy for patients with non-small cell lung cancer: The surgical setting of the Big Lung Trial. Eur J Cardiothorac Surg 2004;26:173–82.
- Tada H, Yasumitu T, Iuchi K, Taki T, Kodama K, Mori T, . Randomized study of adjuvant chemotherapy for completely resected non-small cell lung cancer: Lack of prognostic significance in DNA ploidy pattern at adjuvant setting. Proc Am Soc Clin Oncol 2002;21:Abstract 1250.
- Ueda H, Sakada T, Kuwahara M, Motohiro A. A small randomized phase III single-center trial on postoperative UFT administration in patients with completely resected non-small cell lung cancer. Anticancer Drugs 2004;15:29–33.
- Karrer K, Pridun N, Denck H. Chemotherapy as an adjuvant to surgery in lung cancer. Cancer Chemother Pharmacol 1978;1:145–59.
- Israel L, Bonadonna G, Sylvester R. Controlled study with adjuvant radiotherapy, chemotherapy, immunotherapy and chemoimmunotherapy in operable squamous carcinoma of the lung. In: Muggia F, Rozencweig M, editors. Lung cancer: Progress in therapeutic research. New York: Raven Press; 1979. 443–52.
- Shields TW, Higgins GAJ, Humphrey EW, Matthews MJ, Keehn RJ. Prolonged intermittent adjuvant chemotherapy with CCNU and hydroxyurea after resection of carcinoma of the lung. Cancer 1982;50:1713–21.
- Girling DJ, Stott H, Stephens RJ, Fox W. Fifteen-year follow-up of all patients in a study of post-operative chemotherapy for bronchial carcinoma. Br J Cancer 1985;52:867–73.
- Schallier DC, De Neve WJ, De Greve JL, Van Belle SP, De Wasch GJ, Dotremont G, . Is adjuvant treatment with vinblastine effective in reducing the occurrence of distant metastasis in limited squamous cell lung cancer? A randomized study. Clin Exp Metastasis 1988;6:39–48.
- Trachtenberg A, Zakharchenkov AV, Zhiclov MA. Combined treatment including postoperative chemotherapy in lung cancer patients. Neoplasma 1988;35:351–9.
- The Lung Cancer Study Group. The benefit of adjuvant treatment for resected locally advanced non-small-cell lung cancer. J Clin Oncol 1988;6:9–17.
