To the Editor
Oral tyrosine kinase inhibitors (TKIs) and the monoclonal antibody bevacizumab have significantly changed the management and perspectives of patients with metastatic renal cell cancer (mRCC). Especially patients with a clear cell histological subtype gain benefit from therapies targeted against signalling of the vascular endothelial growth factor (VEGF) and platelet-derived growth factor. Sunitinib has been registered as first- and second-line therapy for mRCC. This TKI can induce high partial response (PR) rates of up to 40% at metastatic sites and can prolong progression-free survival (PFS) and overall survival (OS) as compared to interferon-alpha (11 and 26 months versus 5 and 22 months, respectively) [Citation1]. In primary tumours, sunitinib-induced responses as well as responses induced by the VEGF-directed monoclonal antibody bevacizumab have been described at a rate previously unknown for cytokine-based treatment [Citation2–4]. Therefore, presurgical targeted therapy might facilitate nephrectomy by downsizing primary tumours. In addition, responses have been reported in bulky retroperitoneal lymph nodes and even in tumour thrombi with caval vein extension, which may improve the surgical management of these tumour sites [Citation5–11]. In contrast to these promising reports, we describe two patients with mRCC of a clear cell subtype who developed a progressive caval vein thrombus during sunitinib, which resulted in a negative impact on surgical management.
Case reports
Two patients were treated in accordance with a phase II trial in which sunitinib-induced responses in primary tumours were investigated. The main inclusion criteria of this trial were histologically confirmed mRCC of the clear cell subtype with a resec-table asymptomatic primary tumour in situ, extensive metastatic disease defined as non-resectable metastases in case of one metastatic site or ≥ two metastatic sites, a World Health Organization (WHO) performance status of 0 or 1, an intermediate risk profile according to Memorial Sloan-Kettering Cancer Center (MSKCC) criteria, and no prior systemic treatment with biological response modifiers, TKIs, monoclonal antibodies or chemotherapy. Patients were treated with sunitinib 50 mg/day four weeks on and two weeks off for two cycles. At completion of the second treatment cycle, a computed tomography (CT) scan was performed for response evaluation and patients were admitted for surgery within one week.
Case 1
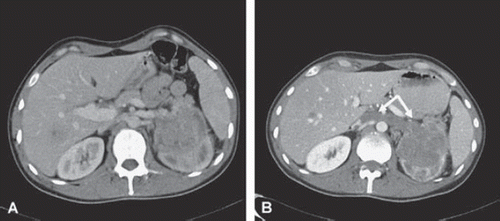
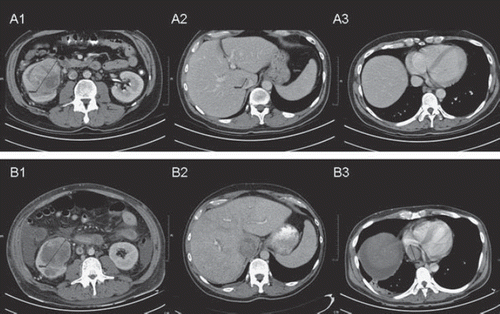
A 49-year-old male patient presented with a primary RCC tumour in the right kidney with thrombus extension into the infrahepatic caval vein up to the liver. The patient had metastatic disease in lungs and para-aortic lymph nodes. Metastatic burden was limited and the sum of the longest diameter of all metastatic lesions was only 2.8 cm. Resection of the primary and thrombus was feasible, but it was decided to include the patient in the protocol and take advantage of potential downsizing of the thrombus. The patient started sunitinib 50 mg/day (four weeks on and two weeks off) as first-line treatment. During the first week of the second cycle the patient was admitted with fatigue grade 3, dyspnoea and ascites. The CT scan revealed extension of the thrombus into the right atrium with liver congestion. The size of the primary tumour and metastases, however, had remained stable (). The performance score deteriorated rapidly from WHO 0 to 3 and nephrectomy could not be performed anymore. The patient died two months after the start of sunitinib due to thrombus-induced backward and liver failure.
Figure 1. CT scans of the first patient at baseline (A) and after (B) one cycle of sunitinib. A1 and B1: The primary tumour has decreased in size. A 2–3 and B 2–3: The caval vein thrombosis has increased in size (A2 and B2) and extends from infrahepatic (A2) towards the right atrium (B3). Note the absence of the thrombus in the right atrium before treatment (A3).
Case 2
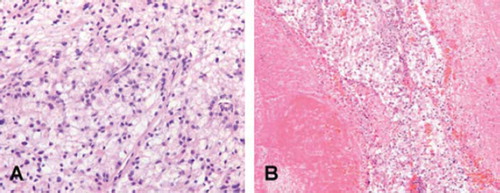
A 35-year-old male patient was diagnosed with resec-table primary RCC in the left kidney, one liver metastasis and bone metastases. After pain-alleviating radiotherapy for metastases in spine and hip, the patient initiated first-line sunitinib treatment at 50 mg/day (four weeks on and two weeks off). At response evaluation in the last week of the second cycle, the liver metastasis showed a minor reduction of 19% in size, while the primary tumour had not changed. In addition, the CT scan revealed a newly developed thrombus in the caval vein (). Nephrectomy was performed in a trans-abdominal approach requiring extension of the surgical field to safely perform a cavotomy. The adjacent tissues to the renal vein were adhesive due to a reaction of the thrombotic material. Histopathological examination demonstrated a primary tumour of 10 cmin diameter with clear cell histology and a necrotic centre. The thrombus contained tumour cells as well (). The patient recovered and continued on sunitinib until progressive disease (PD) in liver and bone lesions five months thereafter. He died from disease after a survival period of 12 months from the time of diagnosis.
Discussion
We here describe two mRCC patients on presurgical sunitinib to facilitate secondary nephrectomy who developed a progressive caval vein thrombus while on treatment. In the first patient, an initially resectable caval vein thrombus progressed into the right atrium and surgery could not be performed due to rapid clinical deterioration as a result from backward and liver failure. In the second patient, a tumour thrombus developed with extension into the caval vein which required extended surgery, while the liver metastasis demonstrated regression. It cannot be excluded that progression of the thrombus in the first patient may have been related to venous thrombotic events [Citation12], but histology revealed a tumour thrombus in the second patient.
In the cytokine era, responses in primary tumours and caval vein thrombi were rare. mRCC patients with primary tumours in situ were more likely to die from distant metastases than from local progression [Citation13]. Therefore, initial cytokine therapy was used to select patients for nephrectomy that showed a response in metastases. For two reasons, however, this strategy may be less logical in mRCC patients treated with targeted agents, such as sunitinib. First, mixed responses in the primary tumour and metastases may occur, indicating the risk of progression of initially resectable primaries to inoperable tumours despite a sunitinib-induced response in metastases. Second, after development of PD during sunitinib and subsequent discontinuation of this drug, a resectable primary tumour may rapidly progress or may cause severe symptoms requiring palliative treatment.
The effect of downsizing primary tumours is most prominent in the first three months, suggesting that a few cycles of sunitinib may be sufficient to facilitate the subsequent surgical procedure. In previous reports on sunitinib, however, the PD rate in primary tumours varied from 0 to 47% [Citation4,Citation14]. The study by Thomas et al. [Citation14] and the present cases indicate that initially resectable primaries and tumour thrombi can progress to inoperable tumours within a few months. The wide range of the PD rate may reflect a high variability in sunitinib sensitivity of primary tumours and indicates that biomarkers are warranted to early identify mRCC patients with failure of downsizing primaries. Since a 24 hour discontinuation period of sunitinib appears to be safe to perform surgery [Citation15], the management plan can be changed rapidly in case patients develop PD in resectable primaries or tumour thrombi in the presence of stable disease in metastases.
Combined therapy of presurgical sunitinib followed by nephrectomy in mRCC patients might improve the quality of life and even prolong PFS and OS. Several patients have been described in whom sunitinib could be discontinued during a disease-free period after this treatment procedure [Citation16]. Since there is no evidence from randomized controlled trials, the role and sequence of cytoreductive surgery in combination with targeted therapy in mRCC are open for debate. Currently, a number of trials have been initiated to investigate the efficacy of presurgical sunitinib in mRCC patients with a primary tumour in situ. The present two cases illustrate, however, that not each patient benefits from this approach. Whether caused by thrombotic events or true tumour growth, caval vein thrombi can develop and progress under targeted therapy. Physicians should be aware of progressive primary tumours and tumour thrombi to avoid missing an opportunity for nephrectomy.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Oudard S, . Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol 2009;27:3584–90.
- Bex A, Van der Veldt AA, Blank C, Van den Eertwegh AJ, Boven E, Horenblas S, . Neoadjuvant sunitinib for surgically complex advanced renal cell cancer of doubtful resectability: Initial experience with downsizing to reconsider cytoreductive surgery. World J Urol 2009;27:533–9.
- Jonasch E, Wood CG, Matin SF, Tu SM, Pagliaro LC, Corn PG, . Phase II presurgical feasibility study of bevacizumab in untreated patients with metastatic renal cell carcinoma. J Clin Oncol 2009;27:4076–81.
- Van der Veldt AA, Meijerink MR, Van den Eertwegh AJ, Bex A, De Gast G, Haanen JB, . Sunitinib for treatment of advanced renal cell cancer: Primary tumor response. Clin Cancer Res 2008;14:2431–6.
- Amin C, Wallen E, Pruthi RS, Calvo BF, Godley PA, Rathmell WK. Preoperative tyrosine kinase inhibition as an adjunct to debulking nephrectomy. Urology 2008;72:864–8.
- Di SF, Sciarra A, Parente U, Andrea A, Von Heland M, Panebianco V, . Neoadjuvant therapy with sorafenib in advanced renal cell carcinoma with vena cava extension submitted to radical nephrectomy. Urol Int 2008;80:451–3.
- Harshman LC, Srinivas S, Kamaya A, Chung BI. Laparoscopic radical nephrectomy after shrinkage of a caval tumor thrombus with sunitinib. Nat Rev Urol 2009;6:338–43.
- Karakiewicz PI, Suardi N, Jeldres C, Audet P, Ghosn P, Patard JJ, . Neoadjuvant sutent induction therapy may effectively down-stage renal cell carcinoma atrial thrombi. Eur Urol 2008;53:845–8.
- Patard JJ, Thuret R, Raffi A, Laguerre B, Bensalah K, Culine S. Treatment with sunitinib enabled complete resection of massive lymphadenopathy not previously amenable to excision in a patient with renal cell carcinoma. Eur Urol 2009;55:237–9.
- Robert G, Gabbay G, Bram R, Wallerand H, Deminiere C, Cornelis F, . Case study of the month. Complete histo-logic remission after sunitinib neoadjuvant therapy in T3b renal cell carcinoma. Eur Urol 2009;55:1477–80.
- Shuch B, Riggs SB, LaRochelle JC, Kabbinavar FF, Avakian R, Pantuck AJ, . Neoadjuvant targeted therapy and advanced kidney cancer: Observations and implications for a new treatment paradigm. BJU Int 2008;102:692–6.
- Zangari M, Fink LM, Elice F, Zhan F, Adcock DM, Tricot GJ. Thrombotic events in cancer patients receiving antiangiogenesis agents. J Clin Oncol 2009;27:4865–73.
- Bex A, Kerst M, Mallo H, Meinhardt W, Horenblas S, De Gast GC. Interferon alpha 2b as medical selection for nephrectomy in patients with synchronous metastatic renal cell carcinoma: A consecutive study. Eur Urol 2006;49:76–81.
- Thomas AA, Rini BI, Lane BR, Garcia J, Dreicer R, Klein EA, . Response of the primary tumor to neoadjuvant sunitinib in patients with advanced renal cell carcinoma. J Urol 2009;181:518–23.
- Margulis V, Wood CG. Cytoreductive nephrectomy in the era of targeted molecular agents: Is it time to consider presurgical systemic therapy? Eur Urol 2008;54:489–92.
- Johannsen M, Florcken A, Bex A, Roigas J, Cosentino M, Ficarra V, . Can tyrosine kinase inhibitors be discontinued in patients with metastatic renal cell carcinoma and a complete response to treatment? A multicentre, retrospective analysis. Eur Urol 2009;55:1430–8.