Abstract
Background. The present study was conducted to evaluate the efficacy and toxicity of a combination of paclitaxel, cisplatin and gemcitabine in patients with carcinoma of unknown primary site (CUP). Patients and methods. Patients with CUP, ECOG performance status 0–1 and age between 18 and 65 years old were treated with paclitaxel 175 mg/m2 day 1, cisplatin 75 mg/m2 day 1 and gemcitabine 1000 mg/m2 day 1 and 8 in a three-week schedule. Results. Ninety-eight patients were enrolled between 1998 and 2008. Ninety-one patients had target lesions according to the RECIST guidelines. The overall response rate was 42.9% (39 patients), including five complete responses (5.5%) and 34 partial responses (37.4%). The median survival time was 10.7 months, and the survival rates at one and two years were 42% and 14%, respectively. The most frequent grade 3 or more adverse events were neutropenia and thrombocytopenia. There were 3 treatment-related deaths. Conclusions. Combination of paclitaxel, cisplatin and gemcitabine is an active regimen in patients with CUP with response and survival rates at least similar to other platinum- and taxane-containing regimens. The treatment was well tolerated by most patients although neutropenia and thrombocytopenia were relatively common. The present regimen represents an attractive regimen in younger CUP patients with a good performance status.
Carcinoma of unknown primary site (CUP) represents a heterogeneous group of metastatic malignancies for which no primary site of the tumour can be identified following a thorough medical history, careful clinical examination and extensive diagnostic work-up. CUP accounts for approximately 5% of all cancer diagnoses and is characterised by early dissemination, uncommon metastatic sites, and usually a poor prognosis [Citation1–3]. In less than 30% of CUP patients a primary site is identified ante mortem. Post mortem examinations reveal a putative primary site in 60–80% of CUP patients, most often in the lung (27%), pancreas (24%) or in the hepatobiliary tree (8%) [Citation4].
Several favourable CUP subsets, representing about 15% of all cases, have been recognised based on specific clinical and pathologic features. These subsets require specific recommended treatment strategies, which may translate into prolonged survival [Citation5]. Unfortunately, the majority of patients with CUP (approximately 85%) do not fit into any of these subsets. As a result, diagnostic and therapeutic strategies for this latter group are less obvious. Failure to identify the primary tumour may negatively influence patient management, as tailored chemotherapeutic regimens and targeted agents have increasingly been developed over the last decade for a number of solid tumours.
The optimal first-line treatment remains to be determined for CUP patients belonging to the unfavourable subset. Various combinations of chemotherapy have been exploited producing response rates as low as 0 and as high as 50% [Citation6]. Taxane/platinum-based chemotherapy regimens have been used in several solid tumours because of their wide spectrum of antineoplastic activity and their moderate toxicity. Gemcitabine as a single agent has shown anti-tumour activity in pancreatic cancer, non-small cell lung cancer (NSCLC) and bladder cancer and with a good tolerability. We designed a phase II study to evaluate the efficacy and tolerability of a combination of paclitaxel, cisplatin and gemcitabine in CUP patients. These agents have a documented effect in lung [Citation7–9] and pancreatic cancer [Citation10,Citation11] , which constitute more than 50% of the primary tumours indicated at autopsy in CUP patients.
Patients and methods
This prospective phase II study was initiated on November 1998 at the Department of Oncology at Copenhagen University Hospital Rigshospitalet, Denmark. The trial was carried out with approval from the local ethics committee, and all patients provided written informed consent.
Eligibility
Inclusion criteria . Patients were considered to have CUP if the following diagnostic procedures were unable to reveal a primary site: (i) thorough history and physical examination; (ii) histology; (iii) chemistry profile, including serum tumour markers in men (prostate specific antigen (PSA), α-fetoprotein (AFP) and β-human gonadotropin (β-HCG)); (iv) X-ray and/or computerised tomography (CT) of the chest and the abdomen; (v) in women ultrasonography (US) and/or magnetic resonance imaging (MRI) of pelvic organs and mammography, and (vi) directed radiological and endoscopic work-up of any symptomatic areas.
Additional eligibility criteria included: (i) no previous chemotherapy; (ii) age between≥18 years and≤65 years; (iii) Eastern Cooperative Oncology Group performance status (PS) 0 or 1; (iv) adequate bone marrow function (granulocytes > 2 × 10 9/l, platelet count > 100 × 10 9/l); (v) normal liver function (bilirubin < 1.5 the institutional upper limits of normal (ULN), and serum alanine transaminase (ALT) and aspartate transaminase (AST) < 3 × ULN, or AST and ALT < 5 × ULN in the presence of liver metastases), and (vi) normal renal function (glomerular filtration ratio (GFR) measured by 51Cr-EDTA clearances≥60 ml/min).
Exclusion criteria. (i) severe cardiac disease; (ii) severe active infection; (iii) other serious medical or psychological factors which might prevent adherence to the treatment schedule; (iv) brain metastases; (v) pre-existing neuropathy; (vi) a history of previous malignancy within five years except non-melanoma skin cancer or in situ carcinoma of the cervix, and (vii) pregnant or lactating women. In addition, favourable subsets were excluded, i.e. (i) women with adenocarcinoma involving only axillary lymph nodes or the peritoneal cavity; (ii) patients with squamous cell carcinoma involving only cervical lymph nodes or inguinal lymph nodes; (iii) patients with poorly differentiated carcinoma consistent with germ cell tumour (i.e. isolated midline structures, multiple pulmonary nodules or elevated levels of β-HCG or AFP); (iv) men with PSA elevated in their plasma or stained in their tumour; (v) patients with neuroendocrine carcinoma, and (vi) patients with single and small, potentially resectable, tumour.
Routine pathologic evaluation included initial light microscopic evaluation and immunohistochemical studies depending on clinical and pathologic features. In patients with poorly differentiated carcinomas it was mandatory to rule out lymphomas (leucocyte common antigen), melanomas (S-100 protein and HMB-45), germ cell tumours (PLAP, CD30, β-HCG and AFP), sarcomas (vimentin and desmin), neuroendocrine tumours (synaptophysin, chromogranin and NSE) and prostate cancer (PSA). Since 2006, a broad panel of antibodies have been applied to all CUP biopsies, which include site-specific antigens with the expression pattern of mucin antigens and intermediary filaments, to help suggesting the primary site in CUP patients.
Treatment protocol
Patients were treated with the following chemotherapy regimen: paclitaxel 175 mg/m2 as a 3-hour intravenous (i.v.) infusion, day 1; cisplatin 75 mg/m2 i.v., day 1, and gemcitabine 1000 mg/m2 as a 30-minute i.v. infusion, day 1 and 8. The doses of the three agents were chosen on the basis of the data from Sørensen et al. in treatment of patients with NSCLC [Citation12], although the regimen was changed from a four-week schedule to a three-week schedule. The treatment was repeated every 21 days for at least four courses if no evidence of tumour progression and the treatment was well tolerated.
During therapy, complete blood cell counts and chemistry profile were monitored weekly. Renal function, measured by 51Cr-EDTA clearance, was performed before every second treatment course. Prior to each course of therapy, adequate haematological function and resolution of any treatment-related non-haematological toxicities to grade 1 or better were required.
Patients were evaluated for response after every two courses of treatment. In patients who obtained at least partial remission (PR) after four courses of therapy, treatment continued until evidence of tumour progression, the development of unacceptable toxicity or consent withdrawal by the patient.
After the completion of therapy, patients were monitored at 2-month intervals until progression of the disease or death. Treatment at the time of progression was at the discretion of the treating physician.
Dose modification
Toxicity was evaluated according to the National Cancer Institute's Common Toxicity Criteria (NCICTC version 2.0) at every course and at the end of treatment. Dose modifications were based on hematological and nonhematological toxicities.
Patients who were hospitalised for the treatment of fever associated with neutropenia received 75% doses of paclitaxel and gemcitabine for all subsequent courses. In case of grade 3 or 4 neurotoxicity, cisplatin was stopped definitively. Paclitaxel was discontinued in patients who experienced severe paclitaxel hypersensitivity reaction. No cisplatin was administered in case of decline in GFR less than 50 ml/min. In case of other organ-specific toxicity, doses were reduced at predefined fractions.
All patient data/files were reviewed for treatment-related toxicity.
Assessment/evaluations
Tumour responses were evaluated after every two courses of chemotherapy and included physical examination, appropriate imaging studies and measurement of clinical/superficial lesions. RECIST guidelines were used to evaluate tumour responses, i.e. complete response (CR), partial response (PR), stable (SD), and progressive disease (PD) [Citation13]. Objective responses (complete and partial responses) required one confirmatory follow-up evaluation at least four weeks after the initial response was determined.
After study completion, an experienced oncoradiologist (KDP) independent of the study reviewed all the CT scans and conventional x-rays in patients with response or stable disease.
In patients with only non-target lesions, the assessment was defined as progressive or non-progressive disease according to the presence of any new lesions.
Statistical analysis
The primary endpoint of this trial was to determine the tumour response rate in patients with CUP treated with a combination of paclitaxel, cisplatin and gemcitabine. Secondary endpoints were toxicity, the duration of response, progression-free survival (PFS) and overall survival (OS). The objective response rate was calculated on intent-to-treat basis among all eligible patients with target lesions/measurable disease who initiated treatment.
Response rates are expressed as percentages with 95% confidence intervals (95% CI). Duration of response, PFS and OS are expressed as the median and 95% CI. Duration of overall response was measured from the time that measurement criteria were first met for CR or PR until the first date that progressive disease or death was documented. PFS was calculated from the first day of treatment to the first sign of PD, last date of follow-up or death. Survival was estimated from the date of treatment initiation until the patient's death.
All data concerning PFS and OS were analysed according to the Kaplan-Meier method by using the SPSS statistical software package (version 15).
The response rate was analysed after inclusion of 29 CUP patients [Citation14]. The overall response rate was 50% and the trial therefore continued.
Results
Patient characteristics
Between November 1998 and February 2008, 98 patients (48 women, 50 men) were enrolled in this clinical trial at Copenhagen University Hospital Rigshospitalet, Denmark. Data were collected until November 2008. Patient characteristics are summarised in . The median age at study entry was 54 years (range: 26–65), and adenocarcinoma was the most common histological diagnosis (68%).
Table I. Patient characteristics (n=98).
Fifty-three of 98 patients (54%) presented with more than two metastatic sites. In case of spread to lymph nodes both above and below the diaphragm, the lymph node metastases were counted as two metastatic sites. Lymph nodes were the most frequent disease localisation (75%), followed by liver (50%), lung (41%) and bones (25%).
Ninety-one patients had measurable lesions in according with the RECIST criteria [Citation13]. Seven patients had only non-target lesions.
Treatment delivered
A total of 535 treatment courses were administered with a median of six courses per patient (range: 1–14). Ten patients received only one course of therapy for the following reasons: clinical evidence of early disease progression (n=3); treatment-related deaths (n=3); intercurrent unrelated illness (n=1); patient refusal (n=1); severe toxicity (n=1) and primary tumour site identified (n=1).
Toxicity
Treatment-related toxicities are listed in . Myelosuppression was the most common toxicity. Grade 3/4 neutropenia occurred in 42.8% of the treatment courses. However, fever associated with neutropenia was seen in only 19 of 535 courses of therapy (3.6%). Most non-hematological toxicities were mild to moderate (grades 1–2).
Table II. Treatment-related toxicities* (No of courses = 535) - rates in brackets.
Dose reductions were necessary in 58 patients, most frequently due to hematological toxicity (20 patients), renal toxicity (24 patients) and/or peripheral neuropathy (20 patients). Cisplatin was discontinued in 12 patients (12%) because of greater than grade 2 neurotoxicity or decline in GFR less than 50 ml/min. Paclitaxel was discontinued in four patients due to hypersensitivity reaction.
There were three treatment-related deaths in this trial caused by febrile neutropenia (n=1) and combination of febrile neutropenia and renal failure (n=2). All treatment-related deaths occurred during the first course of treatment.
Responses and survival
All enrolled patients were analysed for progression-free survival (PFS) and overall survival (OS). Ninety-one patients with target lesions were assessed for response to treatment. In intent-to-treat analysis an objective response was observed in 39 of 91 patients 42.9% (95% CI: 33.1–53.1). Five patients obtained a complete response and 34 patients obtained a partial response. In addition, five patients obtained partial response but the response could not be confirmed because of evidence of progression at the time of the confirmatory scan. Consequently, these patients’ best response were categorised as stable disease. shows responses to the treatment. The median response duration in responders was 8.0 months (95% CI: 5.4–10.6). illustrates the PFS curve for all 98 patients. Five patients changed treatment without progression and these patients are censored at the date of change of treatment. The median PFS duration was 6.6 months (95% CI: 5.2–7.9 months) with a 1-year PFS rate of 15%. Three of the patients who obtained complete response are still alive without progression six, 34 and 37 months after treatment initiation. The three patients were women with only nodal involvement to one or two sites. One patient had lymph node metastases involving the neck and retroperitoneum. The histology was squamous cell carcinoma. The second patient had lymph node metastases in mediastinum. The histology was poorly differentiated carcinoma. The third patient had axillary lymph node metastases. Histology was poorly differentiated carcinoma. Three patients who achieved partial response received additional radical radiotherapy (2 GY × 30) after the end of chemotherapy. Irradiated sites were: mediastinum, retroperitoneum and tumour infiltrating the psoas muscle. Two patients obtained complete response after radiotherapy and are still alive without progression more than five year after treatment was initiated.
Figure 1. Kaplan-Meier estimates of progression-free survival in all enrolled patients (n=98). Median PFS was 6.6 months (95% CI: 5.2–7.9).
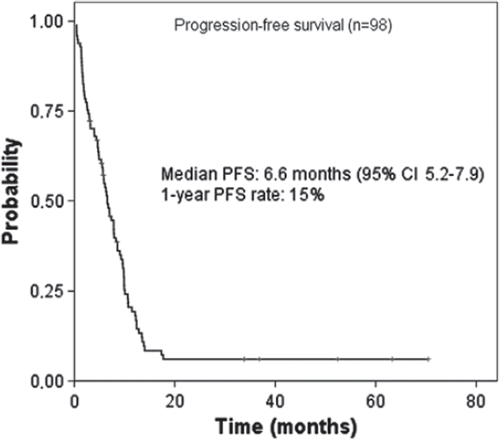
Table III. Responses to therapy for patients with measurable disease (n=91).
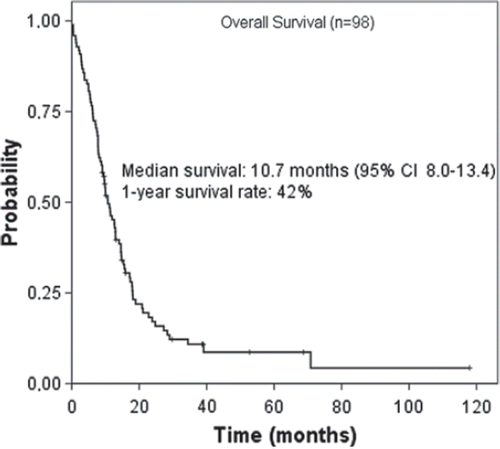
The survival curve for all patients is shown in . The patients were followed from initiation of treatment until death. The duration of follow-up ranged from 11 days to 70 months. The median survival duration was 10.7 months (95% CI: 8.0–13.4), with a 1-year survival rate of 42% and 2-year survival rate of 14%.
Second-line treatment
Forty-six patients (47%) received second-line treatment. The regimens used varied over time and tumour histology.
Discussion
Approximately half of CUP patients have their primary origin either in the pancreas or the lung. Based on this information we designed this prospective phase II study combining paclitaxel, cisplatin and gemcitabine, agents with documented effect in pancreatic and lung cancer [Citation7–11]. At the time this study was initiated, no data were available from other protocols containing a combination of taxane, platinum and gemcitabine. Palmeri et al. [Citation15] published in 2006 the only study using the same combination, and they obtained comparable response rates (48%) and median overall survival (9.6 months).
The therapeutic effects achieved in the present protocol are summarised as a 43% response rate, a median progression-free survival and overall survival rate of 6.6 and 10.7 months, respectively, and 42% of the CUP patients being alive at one year. These results are comparable to most other 2 and 3-drug regimens in CUP patients [Citation6]. To date, fourteen phase II studies have evaluated taxane/platinum combinations in CUP patients with or without a third cytotoxic agent. In addition, six trials have evaluated gemcitabine in combination with either a taxane or platinum. The response rates ranged from 18 to 55% and the median survival times between 6.5 to 16.2 months. The one-year and 2-year survivals varied from 26 to 48% and 7 to 23%, respectively ( and ) [Citation15–34]. 5-Fluorouracil-based regimens have in CUP patients shown lower response rates (mean 22%) and median OS around seven months [Citation35]. It is, however, not possible to draw firm conclusions concerning differences in activity between different studies and regimens for several reasons: (i) most studies are non-randomised phase II trials; (ii) the CUP patient group is heterogeneous and iii) the proportion of patients with poor prognostic factors enrolled may vary among the studies.
Table IV. Two-drug regimens containing platinum, taxane or gemcitabine.
Table V. Three-drug regimens containing platinum, taxane and/or gemcitabine.
Poor prognostic factors have been evaluated and identified in CUP patients and the following variables were found to be inversely correlated with survival: men, performance status greater than one, high number of metastatic sites, presence of liver metastases, elevated serum alkaline phosphatase, elevated lactate dehydrogenase, and low serum albumine [Citation36–38]. Patient selection can therefore have a significant effect on study outcomes. This was shown recently in the trial of Pentheroudakis et al. where patients with predominately nodal disease or women with non-mucinous peritoneal carcinomatosis (favourable subsets) had response rates of 46% and median OS of 22.6 months while the remaining patients belonging to the unfavourable subset had a response rate of 17% and median OS of 5.3 months. The mean response rate for all patients was 32% and the median OS was 16.2 months [Citation31]. In our study, although all enrolled patients had a good performance status (PS = 0 or 1) 86% of the patients had at least one poor prognostic factor, including liver metastases (50% of the patients), more than two metastatic sites (54%), elevated alkaline phosphatase (55%) and/or elevated lactate dehydrogenase (55%).
Paclitaxel, cisplatin and gemcitabine were relatively well tolerated, although grade 3 or 4 neutropenia and thrombocytopenia were common. Neurotoxicity and renal toxicity were troublesome in some patients who continued on study for a prolonged time period, with 12 patients (12%) experiencing greater than grade 2 neurotoxicity or decline in GFR less than 50 ml/min. This was also seen in the study of Sørensen et al., however, no treatment-related death occurred [Citation12]. We experienced three treatment-related deaths (3%), one caused by febrile neutropenia and two caused by febrile neutropenia and renal failure. Two of these patients were admitted to their local hospitals and unfortunately they were not treated according to protocol with the recommended broad-spectrum antibiotics. This may explain the relatively high percentage of treatment-related deaths in this trial.
In conclusion, the described combination of paclitaxel, cisplatin and gemcitabine represents an attractive regimen in younger CUP patients with a good performance status.
Trials combining targeted biological agents and chemotherapy are warranted to make additional improvements in the treatment of CUP patients.
Of note, Hainsworth et al. have shown that targeted agents (bevacizumab and erlotinib) alone or in combination with chemotherapy (carboplatin and paclitaxel) have substantial activity in first-line as well as in second-line treatment of CUP patients [Citation39].
Gene expression profiling may be able to predict the primary site in CUP patients [Citation41–43] and thus enable tailored therapy and hopefully improve survival. Prospective studies are needed to evaluate if site-specific therapy as suggested by gene expression profiling leads to better response and better survival compared to empiric therapy in CUP patients.
Acknowledgements
We would like to thank Kell Osterlind for statistical assistance and Julie Abildgaard for help with collection of patient data.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Pavlidis N, Briasoulis E, Hainsworth J, Greco FA. Diagnostic and therapeutic management of cancer of an unknown primary. Eur J Cancer 2003;39:1990–2005.
- Pavlidis N, Fizazi K. Carcinoma of unknown primary (CUP). Crit Rev Oncol Hematol 2009;69:271–8.
- Randen M, Rutqvist LE, Johansson H. Cancer patients without a known primary: Incidence and survival trends in Sweden 1960–2007. Acta Oncol 2009;48(48):915–20.
- Pentheroudakis G, Golfinopoulos V, Pavlidis N. Switching benchmarks in cancer of unknown primary: From autopsy to microarray. Eur J Cancer 2007;43:2026–36.
- Fizazi K. Treatment of patients with specific subsets of carcinoma of an unknown primary site. Ann Oncol 2006;17(Suppl 10):x177–80.
- Pavlidis N. Forty years experience of treating cancer of unknown primary. Acta Oncol 2007;46:592–601.
- Hotta K, Matsuo K, Ueoka H, Kiura K, Tabata M, Tanimoto M. Meta-analysis of randomized clinical trials comparing Cisplatin to Carboplatin in patients with advanced non-small-cell lung cancer. J Clin Oncol 2004;22:3852–9.
- Scagliotti GV, De MF, Rinaldi M, Crino L, Gridelli C, Ricci S, . Phase III randomized trial comparing three platinum-based doublets in advanced non-small-cell lung cancer. J Clin Oncol 2002;20:4285–91.
- Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, . Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2002;346:92–8.
- Burris HA, III, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, . Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J Clin Oncol 1997;15:2403–13.
- Rivera F, Lopez-Tarruella S, Vega-Villegas ME, Salcedo M. Treatment of advanced pancreatic cancer: From gemcitabine single agent to combinations and targeted therapy. Cancer Treat Rev 2009;35:335–9.
- Sorensen JB, Stenbygaard LE, Dombernowsky P, Hansen HH. Paclitaxel, gemcitabine, and cisplatin in nonresectable non-small-cell lung cancer. Ann Oncol 1999;10: 1043–9.
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, . New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205–16.
- Gothelf A, Daugaard G, Nelausen K. Paclitaxel, cisplatin and gemcitabine in the treatment of unknown primary tumours, a phase II study. Ann Oncol 2002; 13 Suppl 5: abstract 88P.
- Palmeri S, Lorusso V, Palmeri L, Vaglica M, Porta C, Nortilli R, . Cisplatin and gemcitabine with either vinorelbine or paclitaxel in the treatment of carcinomas of unknown primary site: Results of an Italian multicenter, randomized, phase II study. Cancer 2006;107:2898–905.
- Balana C, Manzano JL, Moreno I, Cirauqui B, Abad A, Font A, . Phase II study of cisplatin, etoposide and gemcitabine in an unfavourable group of patients with carcinoma of unknown primary site. Ann Oncol 2003;14:1425–9.
- Berry W, Elkordy M, O'Rourke M, Khan M, Asmar L. Results of a phase II study of weekly paclitaxel plus carboplatin in advanced carcinoma of unknown primary origin: A reasonable regimen for the community-based clinic? Cancer Invest 2007;25:27–31.
- Bouleuc C, Saghatchian M, Di Tullio L, Louvet C, Levy E, Di Palma M, . A multicenter phase II study of docetaxel and cisplatin in the treatment of cancer of unknown primary site (CUP). Proc Am Soc Clin 2001;20:abstact 2300.
- Briasoulis E, Kalofonos H, Bafaloukos D, Samantas E, Fountzilas G, Xiros N, . Carboplatin plus paclitaxel in unknown primary carcinoma: A phase II Hellenic Cooperative Oncology Group Study. J Clin Oncol 2000;18:3101–7.
- Culine S, Lortholary A, Voigt JJ, Bugat R, Theodore C, Priou F, . Cisplatin in combination with either gemcitabine or irinotecan in carcinomas of unknown primary site: Results of a randomized phase II study–trial for the French Study Group on Carcinomas of Unknown Primary (GEFCAPI 01). J Clin Oncol 2003;21:3479–82.
- El-Rayes BF, Shields AF, Zalupski M, Heilbrun LK, Jain V, Terry D, . A phase II study of carboplatin and paclitaxel in adenocarcinoma of unknown primary. Am J Clin Oncol 2005;28:152–6.
- Greco FA, Burris HA, III, Erland JB, Gray JR, Kalman LA, Schreeder MT, . Carcinoma of unknown primary site. Cancer 2000;89:2655–60.
- Greco FA, Erland JB, Morrissey LH, Burris HA, III, Hermann RC, Steis R, . Carcinoma of unknown primary site: Phase II trials with docetaxel plus cisplatin or carboplatin. Ann Oncol 2000;11:211–5.
- Greco FA, Burris HA, III, Litchy S, Barton JH, Bradof JE, Richards P, . Gemcitabine, carboplatin, and paclitaxel for patients with carcinoma of unknown primary site: A Minnie Pearl Cancer Research Network study. J Clin Oncol 2002;20:1651–6.
- Greco FA, Rodriguez GI, Shaffer DW, Hermann R, Litchy S, Yardley DA, . Carcinoma of unknown primary site: Sequential treatment with paclitaxel/carboplatin/etoposide and gemcitabine/irinotecan: A Minnie Pearl Cancer Research Network phase II trial. Oncologist 2004;9:644–52.
- Gross-Goupil M, Fourcade A, Blot F, Penel N, Negrier S, Culine S, . A randomized trial of cisplatin with or without gemcitabine in patients (pts) with carcinoma of an unknown primary (CUP) and without poor prognostic factors: Results of GEFCAPI 02. Ann Oncol 2008;19 Suppl 8: abstract 798P.
- Hainsworth JD, Erland JB, Kalman LA, Schreeder MT, Greco FA. Carcinoma of unknown primary site: Treatment with 1-hour paclitaxel, carboplatin, and extended-schedule etoposide. J Clin Oncol 1997;15:2385–93.
- Huebner G, Link H, Kohne CH, Stahl M, Kretzschmar A, Steinbach S, . Paclitaxel and carboplatin vs gemcitabine and vinorelbine in patients with adeno- or undifferentiated carcinoma of unknown primary: a randomised prospective phase II trial. Br J Cancer 2009;100:44–49.
- Mel JPM, Balaña C, López-Vega J, Casado A, Segura A, Cervera J, . Phase II study of Docetaxel (D), Carboplatin (C) and Gemcitabine (G), in advanced tumors of unknown primary site. J Clin Oncol 2006;4 Suppl 18: abstract 12028.
- Park YH, Ryoo BY, Choi SJ, Yang SH, Kim HT. A phase II study of paclitaxel plus cisplatin chemotherapy in an unfavourable group of patients with cancer of unknown primary site. Jpn J Clin Oncol 2004;34:681–5.
- Pentheroudakis G, Briasoulis E, Kalofonos HP, Fountzilas G, Economopoulos T, Samelis G, . Docetaxel and carboplatin combination chemotherapy as outpatient palliative therapy in carcinoma of unknown primary: A multicentre Hellenic Cooperative Oncology Group phase II study. Acta Oncol 2008;47:1148–55.
- Pittman KB, Olver IN, Koczwara B, Kotasek D, Patterson WK, Keefe DM, . Gemcitabine and carboplatin in carcinoma of unknown primary site: A phase 2 Adelaide Cancer Trials and Education Collaborative study. Br J Cancer 2006;95: 1309–13.
- Pouessel D, Culine S, Becht C, Ychou M, Romieu G, Fabbro M, . Gemcitabine and docetaxel as front-line chemotherapy in patients with carcinoma of an unknown primary site. Cancer 2004;100:1257–61.
- Schneider BJ, El-Rayes B, Muler JH, Philip PA, Kalemkerian GP, Griffith KA, . Phase II trial of carboplatin, gemcitabine, and capecitabine in patients with carcinoma of unknown primary site. Cancer 2007;110:770–5.
- Pentheroudakis G, Greco FA, Pavlidis N. Molecular assignment of tissue of origin in cancer of unknown primary may not predict response to therapy or outcome: A systematic literature review. Cancer Treat Rev 2008;35:221–227.
- Culine S, Kramar A, Saghatchian M, Bugat R, Lesimple T, Lortholary A, . Development and validation of a prognostic model to predict the length of survival in patients with carcinomas of an unknown primary site. J Clin Oncol 2002;20:4679–83.
- Ponce LJ, Segura HA, Diaz BR, Gimenez OA, Aparisi AF, Fleitas KT, . Carcinoma of unknown primary site: Development in a single institution of a prognostic model based on clinical and serum variables. Clin Transl Oncol 2007;9:452–8.
- Seve P, Ray-Coquard I, Trillet-Lenoir V, Sawyer M, Hanson J, Broussolle C, . Low serum albumin levels and liver metastasis are powerful prognostic markers for survival in patients with carcinomas of unknown primary site. Cancer 2006;107:2698–705.
- Hainsworth JD, Spigel DR, Thompson DS, Murphy PB, Lane CM, Waterhouse DM, . Paclitaxel/carboplatin plus bevacizumab/erlotinib in the first-line treatment of patients with carcinoma of unknown primary site. Oncologist 2009;14:1189–1197.
- Hainsworth JD, Spigel DR, Farley C, Thompson DS, Shipley DL, Greco FA. Phase II trial of bevacizumab and erlotinib in carcinomas of unknown primary site: The Minnie Pearl Cancer Research Network. J Clin Oncol 2007;25: 1747–52.
- Bridgewater J, van LR, Floore A, Van't VL. Gene expression profiling may improve diagnosis in patients with carcinoma of unknown primary. Br J Cancer 2008;98:1425–30.
- Horlings HM, van Laar RK, Kerst JM, Helgason HH, Wesseling J, van der Hoeven JJ, . Gene expression profiling to identify the histogenetic origin of metastatic adenocarcinomas of unknown primary. J Clin Oncol 2008;26: 4435–41.
- Varadhachary GR, Talantov D, Raber MN, Meng C, Hess KR, Jatkoe T, . Molecular profiling of carcinoma of unknown primary and correlation with clinical evaluation. J Clin Oncol 2008;26:4442–8.