Abstract
Background. Diagnoses of cancer of the brain, thyroid, eye, bone, and soft tissues are categorised by heterogeneity in disease frequency, survival, aetiology and prospects for curative therapy. In this paper, temporal trends in patient survival in the Nordic countries are considered. Material and methods. Age-standardised incidence and mortality rates, 5-year relative survival, and excess mortality rates for varying follow-up periods are presented, as are age-specific 5-year relative survival by country, sex and 5-year diagnostic period. Results. Brain cancer incidence rates have been rising but mortality has been relatively stable, with 5-year survival uniformly increasing from the early-1970s, particularly in younger patients. Five-year survival from brain cancer among men varies between 45% and 50% for men and 60% to 70% in women, with excess deaths decreasing with time in each of the Nordic populations. Age-standardised incidence rates of thyroid cancer have been mainly increasing during the 1960s and 1970s, although trends thereafter diverge, with 5-year relative survival increasing 20–30 percentage points over the last 40 years to around 80–90%. Thyroid cancer survival is consistently lower in Denmark, particularly in patients diagnosed aged over 60, while there is less geographic variation in excess deaths three months beyond initial diagnosis. Relative survival from eye cancer increased with time from approximately 60% in 1964–1968 to 80% 1999–2003, while for bone sarcoma, incidence rates remained stable, mortality rates declined, and 5-year survival increased slightly to around 55–65%. Soft tissue sarcoma incidence and survival have been slowly increasing since the 1960s, with little variation in survival (around 65%) for the most recent period. Conclusions. There have been some notable changes in survival that can be linked to epidemiological and clinical factors in different countries over time. Time-varying proportions of the major histological subtypes might however have affected the survival estimates for a number of the cancer forms reviewed here.
The neoplasms considered in this article (cancers of the eye, thyroid, bone, soft tissues and tumours of brain and nervous system) comprise five forms variable in terms of disease frequency, survival, aetiology and prospects for curative therapy. As a group they represent about 5% of all cancers in the Nordic countries, with about 60% of these consisting of brain and central nervous system (CNS) tumours, and a further 15% are thyroid cancers.
Brain and CNS tumours represent a relatively uncommon group of neoplasms in terms of incidence, although they do constitute a somewhat more frequent form of cancer death, representing about 3.5% of the total cancer mortality burden [Citation1]. This group is also heterogeneous in that it includes morphologically malignant tumours as well as benign variants, the latter with a behaviour leading to death of the patient. Secular trends in the incidence of, and mortality from these tumours indicate that rates may be increasing in a number of western countries, particularly among the elderly [Citation2], although a bias related to the introduction of new diagnostic imaging techniques cannot be ruled out. Five-year survival in Europe has been modestly increasing in children [Citation3], and in younger adult patients, while 1-year survival has increased irrespective of age [Citation4]. Despite this, survival remains low and the European age-standardised 5-year relative survival was below 20% for persons diagnosed between 1995 and 1999 [Citation5].
Thyroid cancer is also a relatively uncommon form of cancer in the Nordic populations in terms of incidence, where it represents less than 1% of all cancer, and an even less frequent cause of cancer death. The disease comprises a number of distinct histological types with widely-differing clinical and epidemiological features, and is notable for the two- to three-fold higher incidence in women relative to men [Citation6]. Secular trends in incidence (but not mortality) have been increasing in many parts of Europe, including several Nordic countries [Citation7], an observation that reflects in part the introduction of more sophisticated procedures for the detection of thyroid cancer including ultrasonography and fine needle aspiration biopsy, the latter of which has been in use in the Nordic countries since the 1950s [Citation8], and became standard practice in the mid-1970s.
Cancer of the eye is a rare cancer, constituting about 0.3% of all cancers and there were about 300 recorded cases each year in the five countries [Citation1]. More than 80% of all cases are melanomas, and an additional 10% are retinoblastomas [Citation9], with the latter diagnoses commonly restricted to patients aged below 15. There have been few studies of survival, although a recent Danish report of eye melanomas in Denmark 1943–1997 described relative survival as stable over time [Citation10]. Retinoblastomas tend to be associated with high survival, with a survival of 90% or more estimated for a number of European populations [Citation11].
Bone sarcomas are also quite uncommon, comprising around one in 500 of all cancers diagnosed in the Nordic countries each year. They also represent a heterogeneous group of neoplasms for which the most common malignant primary tumours, osteosarcoma, Ewing's sarcoma and chondrosarcoma, vary both epidemiologically and prognostically [Citation12]. Stiller et al. have shown that survival following osteosarcoma and Ewing's sarcoma has increased in patients aged under 20 in recent years [Citation13]. In a previous EUROCARE study of bone sarcoma patients diagnosed 1978–1989, Denmark had slightly lower survival rates than the other participating Nordic countries [Citation14]. A number of hypotheses for differences in the Nordic countries were provided, including heterogeneity in the age distribution at diagnosis, the proportion that were histologically verified, the levels of diagnostic activity, and the degree of specialised care [Citation14].
Despite the relative rarity of soft tissue sarcomas – they comprise approximately one in 150 cancer diagnoses each year in the Nordic countries – they are an important group of childhood cancers. They also constitute a diverse set of histological subtypes, for which the rhabdomyosarcoma and fibrosarcoma are the most common. Histology is an important prognostic factor for this group of patients with fibrosarcoma associated with a higher survival than rhabdomyosarcoma. Improvements in survival for soft tissue sarcoma and for these specific histological groups have been reported in Europe, particularly among children and adolescent patients [Citation15,Citation16].
The aim of the present study is to describe and contrast the country-, sex- and age-specific trends in relative survival and excess mortality of patients diagnosed with each of these cancer types during the diagnostic period 1964–2003 in the five Nordic populations. A further objective is to elucidate these observations by considering concomitant trends in incidence and mortality, alongside relevant clinical and epidemiologic research.
Material and methods
The materials and methods are described in detail in an accompanying article in this issue [Citation17]. In brief, the NORDCAN database contains comparable data on cancer incidence and mortality in the Nordic countries, as delivered by the national cancer registries, with follow-up information on death or emigration for each cancer patient available up to and including 2006. This paper covers the specific cancer sites of the eye (ICD-10 C69), brain and central nervous system (ICD-10 C70-72, D32-33 and D42-43), thyroid (ICD-10 C73), bone (ICD-10 C40-41) and soft tissues (ICD-10 C49 and C46.1). The vast majority of patients diagnosed with these cancers were those of the central nervous system. For the other specified sites, the numbers of cases included for each diagnostic period tend to run in the hundreds, and particular caution should be applied to the very small number of cases generally, and in particular those associated with the statistics generated for Iceland.
We calculated sex-specific 5-year relative survival for each of the diagnostic groups in each country for eight 5-year periods from 1964–1968 to 1999–2003. For the last 5-year period, so-called hybrid methods were used. Country-specific population mortality rates were used for calculating the expected survival. Age-standardisation used the weights of the International Cancer Survival Standard (ICSS) cancer patient populations [Citation18]. We present age-standardised (World) incidence and mortality rates, 5-year relative survival, and excess mortality rates for the follow-up periods of within one month, one to three months and two to five years following diagnosis, as well as age-specific 5-year relative survival by country, sex and 5-year period.
Results
Eye cancer
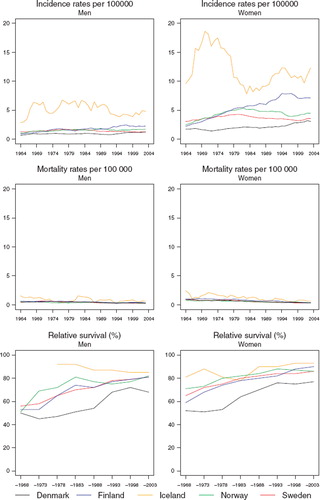
Incidence and mortality. The incidence rate was stable around 1 per 100 000 in each of the Nordic countries for both men and women, with a tendency of higher incidence in Denmark and lower incidence in Sweden (). Mortality following these tumours has been slightly decreasing from 0.4 to 0.2 per 100 000 in the last period in each country. The decrease was more pronounced in Sweden than in the other countries.
Figure 1. Trends in age-standardised (World) incidence and mortality rates per 100 000 and age-standardised (ICSS) 5-year relative survival for eye cancer by sex and country. Nordic cancer survival study 1964–2003.
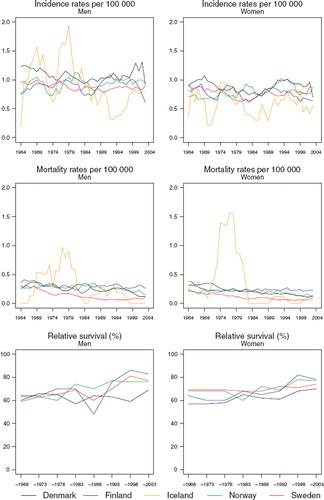
Survival. The relative survival has tended to increase with time, rising from about 60% in the period 1964–1968 to 83% in men and 78% in women for diagnoses 1999–2003, although they are somewhat lower in Denmark (). There is a suggestion of a decline in excess deaths from eye cancer over time in each country, with a larger excess of deaths within the first three months following diagnosis (), although as the underlying numbers are very small, the inherent random variation is such that any interpretation is tentative at best. Survival appears to have risen over time irrespective of age at diagnosis, although again, there is substantive random variation in these estimates ().
Table I. Trends in survival for eye cancer by sex and country. Number of tumours (N) included and the 5-year age-standardised (ICSS) relative survival in percent with 95% confidence intervals (RS (CI)). Nordic cancer survival study 1964–2003.
Table II. Trends in 5-year age-specific relative survival in percent after eye cancer by sex and country. Nordic cancer survival study 1964–2003.
Brain and CNS cancer
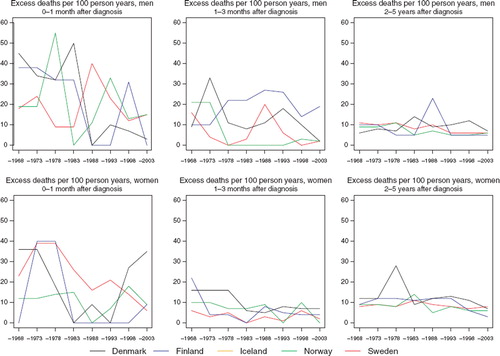
Incidence and mortality. Incidence rates have been increasing in the Nordic countries in the last few decades, rates ranging from 10 to 12 per 100 000 in men and between 11 and 13 in women circa 2000. The upwards trends are perhaps more apparent in the 1970s and 1980s, but in some countries – most evident in Sweden – recent declines have been observed (). In contrast, incidence rates in Norway have been increasing rapidly in the last decade. Mortality rates are however relatively stable with time, at least from the early-1970s with a level around four to six deaths per 100 000.
Figure 3. Trends in age-standardised (World) incidence and mortality rates per 100 000 and age-standardised (ICSS) 5-year relative survival for cancers of the brain & central nervous system by sex and country. Nordic cancer survival study 1964–2003.
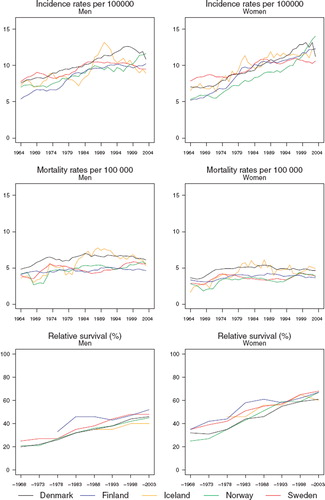
Survival. The 5-year survival of patients diagnosed with brain and CNS tumours has been uniformly increasing over time in all Nordic countries since the early-1970s. The magnitude of change in the trends with time is similar between the sexes, although 5-year survival proportions are evidently higher among female patients. Five-year survival among men in the Nordic patient populations varies between 40% and 50% compared with an approximate range of 60 to 70% in women (). The absolute increases in 5-year survival are of the order 20 to 30 percentage points in men and women respectively over the diagnostic period 1964 to 2003.
Table III. Trends in survival for cancer of the brain & nervous system by sex and country. Number of tumours (N) included and the 5-year age-standardised (ICSS) relative survival in percent with 95% confidence intervals (RS (CI)). Nordic cancer survival study 1964–2003.
Excess deaths per 100 person years have decreased over time in each of the Nordic populations (). Since the early-1980s the excess mortality in the first month after diagnosis has been rather stable. Excess deaths tend to be close one to three months after diagnosis, although Denmark historically has a greater number of excess deaths. There are no apparent country-specific differences in the excess deaths beyond three months after diagnosis (data partially shown). While the increasing survival over time is seen across all ages, the absolute increases tend to be greater and more consistent among patients age groups 0–49 and 50–59 ().
Figure 4. Trends in age-standardised (ICSS) excess death rates per 100 person years for cancers of the brain & central nervous system by sex, country, and time since diagnosis in Nordic cancer survival study 1964–2003.
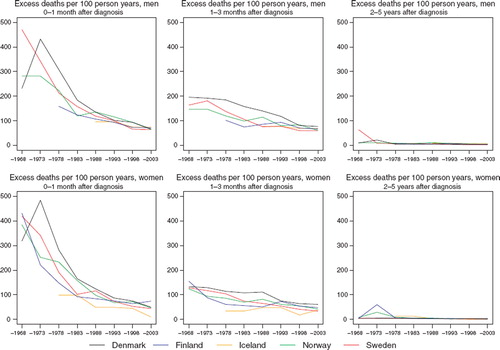
Table IV. Trends in 5-year age-specific relative survival in percent after cancer of the brain & nervous system by sex and country. Nordic cancer survival study 1964–2003.
Thyroid cancer
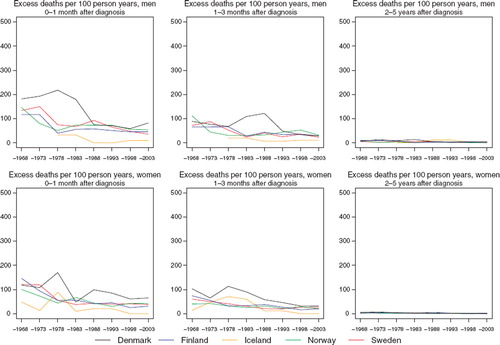
Incidence and mortality. Age-standardised incidence rates of thyroid cancer have been slightly increasing in the Nordic populations in both sexes during the 1960s and 1970s, possibly with the exception of Denmark. Trends thereafter tend to diverge (). In Finland and Denmark, incidence rates increased during the 1980s but appear to have stabilised more recently. In contrast, rates in Norway and Sweden have been in long-term decline over the last 25 years. It is evident that the country-specific trends in incidence are quite similar in men and women albeit with a consistent two to three-fold higher rate observed among women relative to men and much larger absolute difference between the countries for women. Icelandic men and women have incidence rates currently around three times higher than elsewhere. The rates are substantially higher in women during the late-1960s through to the mid-1970s. Trends in mortality have, in contrast, been largely decreasing in both men and women (more decipherable on a semi-log scale of ), with the declines tending to be consistent across countries since the early-1980s. The mortality rates have decreased from around 0.9 and 0.5 per 100 000 for men and women respectively in the early-1960s to about 0.3 for both sexes presently.
Figure 5. Trends in age-standardised (World) incidence and mortality rates per 100 000 and age-standardised (ICSS) 5-year relative survival for thyroid cancer by sex and country. Nordic cancer survival study 1964–2003.
Survival. Age-standardised 5-year relative survival following thyroid cancer has increased 20–30 percentage points over the last 40 years in all Nordic countries and in both sexes to around 80–90%, although survival estimates are consistently lower in Denmark irrespective of the study period (). The absolute changes in the survival proportions with time are reasonably similar between countries, particularly during the last 15 years. With the exception of Denmark, recent survival proportions are quite close between countries in absolute terms, with women displaying a clear survival advantage of around 5–10 percentage points over men, an observation that is not restricted to the latest period of diagnosis ().
Table V. Trends in survival for thyroid cancer by sex and country. Number of tumours (N) included and the 5-year age-standardised (ICSS) relative survival in percent with 95% confidence intervals (RS (CI)). Nordic cancer survival study 1964–2003.
Lower thyroid cancer survival ratios in Denmark are seen irrespective of age and period of diagnosis, although the major differences appear to relate more to the poorer prognosis of patients aged 60 and over (). The observed overall pattern in all countries is that survival decreases with high age. Danish patients also have a higher excess mortality among patients within the first three months of diagnosis (). Within the first three months the Danish excess mortality has been reduced to about 50 per 100 person years circa 2000. There appears to be little variation in the Nordic countries when one considers excess deaths occurring three months after initial diagnosis (data partially shown).
Table VI. Trends in 5-year age-specific relative survival in percent after thyroid cancer by sex and country. Nordic cancer survival study 1964–2003.
Bone sarcoma
Incidence and mortality. The age-standardised incidence rates of bone sarcoma have been rather stable over time in each of the Nordic countries in both sexes (), although in Finland, rates have been slowly but steadily declining since the beginning of the observation period, while in Norway, incidence appears to be gradually rising, particularly among women since the 1970s. The overall incidence rate in the Nordic countries is below 1 per 100 000 in men and slightly lower in women, at around 0.7. Long-term declines in mortality rates are seen in all countries, with little variation in absolute terms in bone sarcoma mortality circa 2000 between populations, or according to sex. The relative magnitude of these decreasing trends appears to be greater than that seen for incidence. Mortality from bone sarcoma is now at a similar level in the Nordic countries at about 0.4 per 100 000 in men and 0.3 in women.
Figure 7. Trends in age-standardised (World) incidence and mortality rates per 100 000 and age-standardised (ICSS) 5-year relative survival for bone sarcoma by sex and country. Nordic cancer survival study 1964–2003.
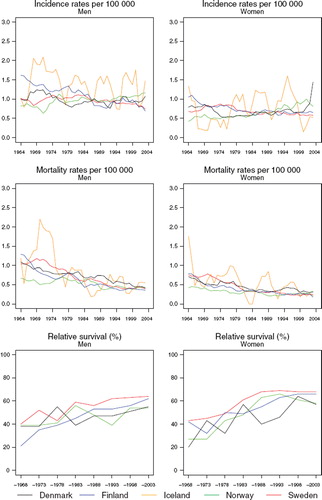
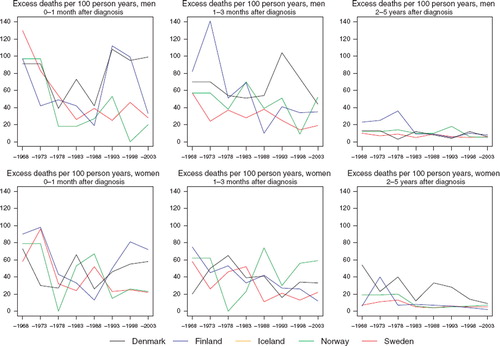
Survival. While trends in 5-year relative survival display some variability, they are generally upwards in each Nordic population (). Survival proportions were of the order 55–65% for patients diagnosed between 1999 and 2003 in the Nordic countries, although survival is a few percentage points higher in women. Survival from bone sarcoma in Sweden is higher than the other countries in both sexes, an observation that is evident in survival comparisons dating back to around 1980 (). The steep progression of survival proportions seen in Norwegian women until around 1990 has since shifted downwards but there is uncertainty in these figures due to sparse data. The excess death figures are also difficult to interpret in light of the inherent random variation associated with such a rare cancer, although it does not appear that there is much variation with regards to prognosis across the Nordic populations, irrespective of diagnostic period (). The survival trends by age indicate increasing survival in all Nordic countries irrespective of age at diagnosis in both sexes, with the absolute increases in survival most notable in the youngest patients, aged under 50 ().
Table VII. Trends in survival for bone sarcoma by sex and country. Number of tumours (N) included and the 5-year age-standardised (ICSS) relative survival in percent with 95% confidence intervals (RS (CI)). Nordic cancer survival study 1964–2003.
Table VIII. Trends in 5-year age-specific relative survival in percent after bone sarcoma by sex and country. Nordic cancer survival study 1964–2003.
Soft tissue sarcoma
Incidence and mortality. In general, soft tissue sarcoma incidence has been slowly increasing among men and women in each of the Nordic populations since the 1960s, although there appear to be sex-specific inconsistencies in the trends in the last decade (). Incidence is around 2–2.5 per 100 000 among males in all Nordic countries and slightly below two in females circa 2000, with rates highest in Finland. Trends in mortality are difficult to decipher given the random variation in the time trends but they tend to be either relatively stable or weakly declining in recent years.
Figure 9. Trends in age-standardised (World) incidence and mortality rates per 100 000 and age-standardised (ICSS) 5-year relative survival for soft tissue sarcoma by sex and country. Nordic cancer survival study 1964–2003.
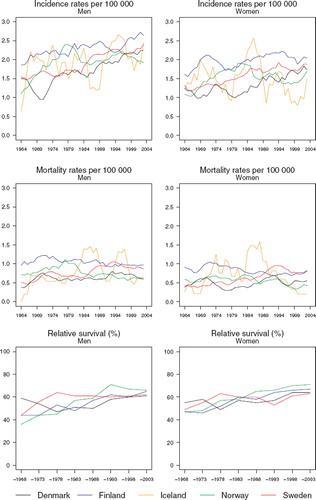
Survival. Gradual increases in 5-year relative survival are suggested in both men and women throughout the period of study, possibly with the exception of Sweden, where trends have been rather stable during the last 30 years (). Survival is consistently higher in Norway than elsewhere in both sexes during the most recent 15 years. There is however little difference in overall survival in the most recent period of diagnosis 1999–2003, which varies around 65% (). While prognosis of patients within the first three months of diagnosis has improved with time, there is no consistent observation of a higher excess mortality in any one or more Nordic countries (). The variation in excess deaths between the Nordic populations has been largely removed two to five years after initial diagnosis. Survival proportions have increased across all ages, although estimates are highest in the group of patients aged 0–49 at diagnosis ().
Figure 10. Trends in age-standardised (ICSS) excess death rates per 100 person years for soft tissue sarcoma by sex, country, and time since diagnosis in Nordic cancer survival study 1964–2003. No Icelandic curves. Too few partients to calculate survival in Iceland.
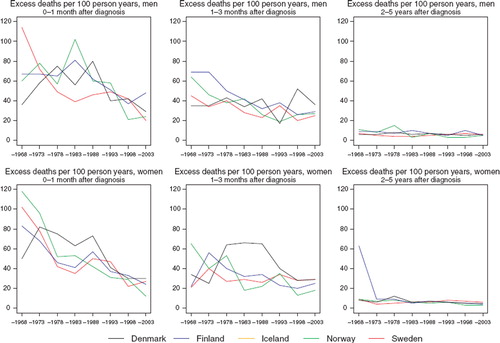
Table IX. Trends in survival for soft tissue sarcoma by sex and country. Number of tumours (N) included and the 5-year age-standardised (ICSS) relative survival in percent with 95% confidence intervals (RS (CI)). Nordic cancer survival study 1964–2003.
Table X. Trends in 5-year age-specific relative survival in percent after soft tissue sarcoma by sex and country. Nordic cancer survival study 1964–2003.
Discussion
This study has attempted to summarise the Nordic patient survival experience following diagnoses of a diverse set of specified, but relatively uncommon cancer sites. The text that follows provides, for each of the neoplasms considered, a synopsis of the key findings and some commentary on their interpretation in relation to the relevant epidemiological and clinical literature.
Brain and CNS cancer constitute a heterogeneous group of histologic types, and there is, at present, a paucity of explanatory factors for the upsurge in brain and CNS incidence in recent decades in the Nordic countries. The lack of decline in mortality with time would suggest that part of the increase in overall brain and CNS survival is artefact related to increasing diagnostic intensity with calendar time. Part of the increase in incidence may be explained by increasing use of, and improvements in, diagnostic techniques, as well as therapeutic or high-dose radiation, the only firmly-established risk determinant [Citation19]. There are several candidate explanations for the decreasing incidence trend in Sweden since the early-1980s, including a reduced autopsy intensity among the elderly, underreporting [Citation20], or an age-specific differential in the use of new imaging techniques. In the latter instance, a tendency to screen younger age groups with symptoms may have led to a subsequent decline in overall incidence.
Survival is generally poorer in adults than in children, and prognosis tend to worsen with age; other survival determinants include performance status and tumour histology [Citation21–23]. The extent of resection appears to be the most consistent prognostic factor in patients with high-grade glioma [Citation24].
The brain and CNS survival proportions in the Nordic countries at five years are higher than in other patient series [Citation4,Citation22], but it is unclear whether progress in imaging and treatment (surgery, chemotherapy and radiotherapy) will have improved childhood and adult CNS tumour survival [Citation3]. The female advantage regarding 5-year survival seen in this study is in line with other European countries as shown based on EUROCARE-4 data [Citation25]. There may however have been some modest survival improvements among patients diagnosed with certain histological subtypes, including medulloblastoma and low-grade and unbiopsied gliomas [Citation4,Citation22].
Interpretation of the geographical and temporal variations of brain and CNS tumours is however beset by potential artefactual concerns. The group represents a vast array of different histological subtypes with varying prognosis. Incidence is heavily influenced by local registration practices concerning the inclusion of benign tumours, unspecified tumours and those of unspecified behaviour, although benign brain tumours are included in the incidence in each of the Nordic registries. The introduction of new technologies including CT scanning and MRI will have improved detection rates over the last 30 years and will certainly explain part of the observed increases in incidence (and probably survival) in the Nordic countries. A degree of underreporting to registries of cancers of the brain and CNS is also possible, but this may be largely restricted to unbiopsied tumours [Citation26].
Mortality rates of thyroid cancer have been decreasing in all Nordic countries during the last 25 years while the 5-year relative survival has been steadily increasing over the same period, leading to an overall survival for patients diagnosed 1999–2003 at 80–90%, an estimate that may be considered quite favourable. Denmark appears to have poorer survival than elsewhere, although the underlying reasons for this disparity and the overall survival improvements are difficult to gauge. A number of factors may be in operation, and the absence of key additional information on, for example, histology and staging limits the depth of the survival analysis and subsequently the scope of our interpretation.
Thyroid cancer incidence tended to increase during the 1960s and 1970s in each population, but rates subsequently stabilised or declined. That rates in Icelandic females exhibited very rapid but transitory increases in the early-1970s lend support to the presence of diagnostic artefact in the numerator, since a very high percentage were diagnosed at incidental autopsy and thus excluded from the survival calculations. Alternatively, the consistently higher rates in both sexes relative to other Nordic regions might additionally imply that levels of background risk are higher in Iceland than elsewhere. Results from Nordic epidemiological studies that have sought to identify the specific determinants have been inconsistent and in short supply.
Accompanying the trends in thyroid cancer incidence rates, population-based patient survival has risen throughout Europe and in the Nordic countries in recent decades [Citation27,Citation28]. Temporal and geographical differences in overall thyroid cancer survival remain difficult to interpret however without further stratification by histological subtype and extent of disease. Heterogeneity in diagnostic intensity, as well as changing histological composition will likely influence the magnitude of these survival estimates, and accordingly, the utility of comparisons between countries and over time. A differential in the distribution of histological subgroups has been shown to be a key prognostic factor for thyroid cancer [Citation27–29]. Prognosis ranges from excellent for the most common form, papillary cancer, to rather poor for poorly-differentiated and anaplastic carcinomas. Colonna and colleagues have speculated that the superior survival observed recently in Norway results from a higher proportion of papillary cancers among its cases (an observation made in 1983 on the basis of a Finland comparison [Citation30]), and in general, that better survival in Europe is seen where the proportion of papillary cancers is highest [Citation28]. This phenomenon may explain the higher survival in women, relative to men [Citation25,Citation31] but other explanations – such as a role for sex hormones – have been postulated [Citation32].
Another possible reason for the observed survival differences may relate to a differential in the use of new diagnostic techniques in the Nordic countries that have improved the ability to diagnose thyroid cancer. Improved fine-needle aspiration techniques and interpretation, along with thyroid ultrasonography has likely increased the proportion of microcarcinomas which may have subsequently led to improvements in survival where practice is more intensive [Citation28].
Both of these factors may partially explain the thyroid cancer survival differentials and in particular, the lower survival from thyroid cancer in Denmark. Treatment for differentiated tumours has not changed appreciably in the last decades [Citation6], although TNM staging including lymph node and distant metastases and complete removal of the tumour in operated patients are significant prognostic factors in Sweden [Citation29]. Differences in stage of presentation and the quality of surgery across the Nordic countries may thus be an important marker of survival differentials. Finally, it has been shown that smokers appear to have an inferior survival following a thyroid cancer diagnosis than non-smokers [Citation29]; given the differentially high prevalence of current smokers in Denmark [Citation33], this factor may be of additional relevance to overall prognosis following a thyroid cancer diagnosis.
For cancers of the eye, mortality rates have fallen moderately while survival has increased. The improved survival in the younger age group was probably the result of improving prognosis among the retinoblastoma group, although a Danish study has reported increases in survival of patients diagnosed with eye melanomas at younger ages [Citation10]. In that study the diagnostics did not appear to change over the period and the changes in treatment did not appear to affect the survival, echoing a Swedish study showing an improved survival of eye cancer patients with calendar time but for unexplained reasons [Citation34].
In simple terms, incidence and mortality rates of bone sarcoma have tended to decline over time in the Nordic countries, irrespective of sex and population, with the downturn in rates seemingly more rapid for mortality. The reduction in case fatality and increases in survival may relate to clinical improvements, but artefactual explanations might also contribute. In Finland, for example, the proportion of unknown histology has uniformly declined with time, and it has been reported that this group probably included a large number of bone metastases of other tumours in early periods [Citation35]. Storm has linked the Nordic differences to the degree of availability of specialist centres for the referral and treatment of patients, citing lower recurrence rates as a possible reason for the higher survival proportions in Sweden [Citation14]. Improving treatment, particularly in children and young adults may be a partial explanation, and is supported by the greatest survival increases observed in the youngest age group in this study. Stiller et al. have shown that bone sarcoma survival has increased in both children and adolescents in Europe, citing local treatment in combination with systemic chemotherapy for treating osteosarcoma and Ewing's sarcoma as a possible explanation [Citation13]. Female gender has been shown to be a strong predictor of favourable outcome in patients with classical osteosarcoma [Citation36].
As with other cancers reviewed in this article (cancers of the brain, thyroid, and soft tissues), the effect of an improving ability to diagnose bone sarcomas over time, and the potential artificial improvements in survival cannot be excluded. A changing distribution of histological types between countries over time may also have had an impact on survival, where, for example, the relative proportion of chondrosarcomas, tumours associated with rather good prognosis, differed. Finally, a high excess mortality 0–3 months after diagnosis was less apparent in Denmark for bone sarcoma in men, although survival was somewhat lower than elsewhere. This might imply a sex-specific differential in factors other than stage at presentation and initial treatment are of importance in explaining the survival differences for certain bone lesion subtypes, but further in-depth analyses examining stage and histology are warranted.
From a European perspective, 5-year relative survival among patients diagnosed with bone sarcoma from the Nordic countries is in line with that of patients from UK and parts of Western Europe (Belgium, Austria, France). Sant and colleagues also showed that 5-year survival, conditional on surviving one year, among Danish patients was higher than in the other Nordic countries [Citation5].
There have been few population-based studies investigating the survival of patients with soft tissue sarcoma. The survival increases seen across Nordic countries in both sexes may suggest improving treatment as a possible factor. Tumour size, histology, primary site, grade and the presence of metastasis have been reported as key prognostic factors in treating the disease [Citation37]. In a recent paper on childhood cancer survival, the 5-year survival of children increased to 74% in the Nordic countries (excluding Sweden) for the period 1993–1997, while corresponding survival for fibrosarcoma was 89% and for rhabdomyosarcoma, 67% [Citation38]. Improvements in the latter subtype have been ascribed to advancements in therapeutic options including better surgery and chemotherapy. The degree of specialisation of care may also be important [Citation14].
Soft tissue sarcoma incidence and survival trends are however difficult to interpret given the low rates, and there are further difficulties in definition and the poor reproducibility of histological subtypes [Citation39]. As with other cancers reviewed here, changing proportions of the major histological subtypes in the different countries over time might affect the overall survival estimates, as could a growing interest and ability to diagnose these tumours in consecutive calendar periods.
Acknowledgement The Nordic Cancer Union (NCU) has financially supported the development of the NORDCAN database and program, as well as the survival analyses in this project.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Engholm G, Ferlay J, Christensen N, Bray F, Klint Å, Ólafsdóttir E, . NORDCAN: Cancer incidence, mortality and prevalence in the Nordic countries, Version 3.3. http://www.ancr.nu 2008.
- Muir CS, Storm HH, Polednak A. Brain and other nervous system tumours. Cancer Surv 1994;19–20:369–92.
- Magnani C, Aareleid T, Viscomi S, Pastore G, Berrino F. Variation in survival of children with central nervous system (CNS) malignancies diagnosed in Europe between 1978 and 1992: The EUROCARE study. Eur J Cancer 2001;37: 711–21.
- Sant M, van der Sanden G, Capocaccia R. Survival rates for primary malignant brain tumours in Europe. Eur J Cancer 1998 1;34:2241–7.
- Sant M, Allemani C, Santaquilani M, Knijn A, Marchesi F, Capocaccia R. EUROCARE-4. Survival of cancer patients diagnosed in 1995–1999. Results and commentary. Eur J Cancer 2009;45:931–91.
- Hall P, Adami HO. Thyroid cancer. Adami HO, Hunter D, Trichopolous D. Textbook of cancer epidemiology 2nd Oxford: Oxford University Press; 2008. 188–211.
- Kilfoy BA, Zheng T, Holford TR, Han X, Ward MH, Sjodin A, . International patterns and trends in thyroid cancer incidence, 1973–2002. Cancer Causes Control 2009; 20:525–31.
- Soderstrom N. Puncture of goiters for aspiration biopsy. Acta Med Scand 1952;144:237–44.
- Curado MP, Edwards B, Shin HR, Storm H, Ferlay J, Heanue M, . Cancer incidence in five continents Vol. IX (IARC Scientific Publication No 160). Lyon: IARC; 2007.
- Isager P, Engholm G, Overgaard J, Storm H. Uveal and conjunctival malignant melanoma in Denmark 1943–97: Observed and relative survival of patients followed through 2002. Ophthalmic Epidemiol 2006;13:85–96.
- MacCarthy A, Draper GJ, Steliarova-Foucher E, Kingston JE. Retinoblastoma incidence and survival in European children (1978–1997). Report from the Automated Childhood Cancer Information System project. Eur J Cancer 2006;42: 2092–102.
- Miller RM, Boice JD, Curtis RE. Bone cancer. Cancer epidemiology and prevention 3rd New York: Oxford University Press; 2006.
- Stiller CA, Bielack SS, Jundt G, Steliarova-Foucher E. Bone tumours in European children and adolescents, 1978–1997. Report from the Automated Childhood Cancer Information System project. Eur J Cancer 2006;42:2124–35.
- Storm HH. Survival of adult patients with cancer of soft tissues or bone in Europe. Eur J Cancer 1998;34:2212–7.
- Pastore G, Peris-Bonet R, Carli M, Martinez-Garcia C, Sanchez DT, Steliarova-Foucher E. Childhood soft tissue sarcomas incidence and survival in European children (1978–1997): Report from the Automated Childhood Cancer Information System project. Eur J Cancer 2006;42: 2136–49.
- Stiller CA, Stevens MC, Magnani C, Corazziari I. Survival of children with soft-tissue sarcoma in Europe since 1978: Results from the EUROCARE study. Eur J Cancer 2001; 37:767–74.
- Engholm G, Gislum M, Bray F, Hakulinen T. Trends in the survival of patients diagnosed with cancer in the Nordic countries 1964–2003 followed up to the end of 2006. Material and methods. Acta Oncol; 2010.
- Corazziari I, Quinn M, Capocaccia R. Standard cancer patient population for age standardising survival ratios. Eur J Cancer 2004;40:2307–16.
- Savitz DA, Trichopolous D. Brain cancer. Adami HO, Hunter D, Trichopolous D. Textbook of cancer epidemiology 2nd. Oxford: Oxford University Press; 2008. 188–211.
- Barlow L, Westergren K, Holmberg L, Talback M. The completeness of the Swedish Cancer Register: A sample survey for year 1998. Acta Oncol 2009;48:27–33.
- Curran WJ, Jr., Scott CB, Horton J, Nelson JS, Weinstein AS, Fischbach AJ, . Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst 1993;85: 704–10.
- Davis FG, Freels S, Grutsch J, Barlas S, Brem S. Survival rates in patients with primary malignant brain tumors stratified by patient age and tumor histological type: An analysis based on Surveillance, Epidemiology, and End Results (SEER) data, 1973–1991. J Neurosurg 1998;88:1–10.
- Shugg D, Allen BJ, Blizzard L, Dwyer T, Roder D. Brain cancer incidence, mortality and case survival: Observations from two Australian cancer registries. Int J Cancer 1994;59: 765–70.
- Buckner JC. Factors influencing survival in high-grade gliomas. Semin Oncol 2003;30(6 Suppl 19):10–4.
- Micheli A, Ciampichini R, Oberaigner W, Ciccolallo L, de Vries E, Izarzugaza I, . The advantage of women in cancer survival: An analysis of EUROCARE-4 data. Eur J Cancer 2009;45:1017–27.
- Johannesen TB, Langmark F, Lote K. Cause of death and long-term survival in patients with neuro-epithelial brain tumours: A population-based study. Eur J Cancer 2003;39: 2355–63.
- Teppo L, Hakulinen T. Variation in survival of adult patients with thyroid cancer in Europe. EUROCARE Working Group. Eur J Cancer 1998;34(14 Spec No):2248–52.
- Colonna M, Grande E, Jonasson JG, Eurocare Working Group. Variation in relative survival of thyroid cancers in Europe: Results from the analysis on 21 countries over the period 1983–1994 (EUROCARE-3 study). Eur J Cancer 2006;42:2598–608.
- Lundgren CI, Hall P, Dickman PW, Zedenius J. Clinically significant prognostic factors for differentiated thyroid carcinoma: A population-based, nested case-control study. Cancer 2006;106:524–31.
- Hakulinen T. A comparison of nationwide cancer survival statistics in Finland and Norway. World Health Stat Q 1983;36:35–46.
- Sant M, Aareleid T, Berrino F, Bielska LM, Carli PM, Faivre J, . EUROCARE-3: Survival of cancer patients diagnosed 1990–94--results and commentary. Ann Oncol 2003;14(Suppl 5):v61–118.
- Lundgren CI, Hall P, Ekbom A, Frisell J, Zedenius J, Dickman PW. Incidence and survival of Swedish patients with differentiated thyroid cancer. Int J Cancer 2003;106:569–73.
- Forey B, Hamling J, Lee P, Wald N. International smoking statistics. A collection of historical data from 30 economically developed countries, 2nd. London & Oxford: Wolfson Institute of Preventive Medicine and Oxford University Press; 2002.
- Bergman L, Nilsson B, Ragnarsson-Olding B, Seregard S. Uveal melanoma: A study on incidence of additional cancers in the Swedish population. Invest Ophthalmol Vis Sci 2006;47:72–7.
- Hakulinen T. Sarkoomien epidemiologia (in Finnish). Focus Oncologiae 2006;7:8–13.
- Smeland S, Muller C, Alvegard TA, Wiklund T, Wiebe T, Björk O, . Scandinavian Sarcoma Group Osteosarcoma Study SSG VIII: Prognostic factors for outcome and the role of replacement salvage chemotherapy for poor histological responders. Eur J Cancer 2003; 39:488–94.
- Swallow CJ, Catton CN. Local management of adult soft tissue sarcomas. Semin Oncol 2007;34:256–69.
- Pastore G, Peris-Bonet R, Carli M, Martinez-Garcia C, Sanchez de Toledo J, Steliarova-Foucher E. Childhood soft tissue sarcomas incidence and survival in European children (1978–1997): Report from the Automated Childhood Cancer Information System project. Eur J Cancer 2006;42:2136–49.
- Berwick M. Soft tissue sarcoma. Cancer epidemiology and prevention. 3rd. New York: Oxford University Press; 2006.