Abstract
Background. Hodgkin lymphoma, Non-Hodgkin lymphoma, multiple myeloma, and acute and other leukaemias constitute about 7% of the overall cancer incidence and 8% of cancer mortality in the Nordic countries. The aim of this study is to describe and interpret the trends in relative survival and excess mortality in the five Nordic populations among these patients. Material and methods. Using the NORDCAN database 1964–2003, we estimated age-standardised incidence and mortality rates, 5-year relative survival, and excess mortality rates for varying follow-up periods, and age-specific 5-year relative survival by country, sex, and 5-year diagnostic period. Results. Taking into account classification and registration problems in the earlier periods, the patterns of incidence, mortality, and survival are fairly similar between the countries within each cancer form studied. High 5-year relative survival ratios of over 80% were seen in the most recent period 1999–2003 for Hodgkin lymphoma, between 50 and 60% for Non-Hodgkin lymphoma, 38–49% for acute leukaemia and 60–73% for other leukaemia. The variations were between 28 and 41% for multiple myeloma. Danish patients diagnosed with these malignancies tend to fare slightly worse than their Nordic neighbours, with excess mortality rates marginally higher one to three months after diagnosis. Conclusion. Although the recent trends and absolute levels of incidence, mortality and survival for the lympho-haematopoietic malignancies are similar, the consistently lower survival of Danish patients – irrespective of type of malignancy – points to an impact of co-morbidity related lifestyle factors, which may negatively affect the chemotherapy and radiation offered as standard treatments for these diseases.
The lymphoid and haematopoietic malignancies constituted 7.4% of the incidence and 8.5% of the mortality in the Nordic countries in 2003 [Citation1]. These neoplasms are rarely localised and have from the beginning of cancer registration activities been subject to classification and coding problems, and hence population-based comparisons have been difficult to conduct at a satisfactory level from both clinical as well as epidemiological perspectives.
Lymphomas have traditionally been grouped into two broad but distinct categories, Hodgkin lymphoma (HL) (0.5% of all cancers) and non-Hodgkin lymphoma (NHL) (3.3%). Some NHL cases may in the past have been misclassified as HL, but with advances in diagnostics, such cases have become increasingly more likely to be correctly classified as NHL. The histological classification of NHL has evolved over many decades, and while over 30 different groupings have been proposed for the study of prognosis and treatment, such categorisations have had limited potential in the study of the aetiological determinants. Risk factors include viral infections, immunodeficiency and immunosuppression, radiation, drugs, and occupational exposure to chemicals, although the aetiology of most NHL remains largely unknown [Citation2,Citation3]. For HL, Epstein Barr virus (EBV) is a likely cause for some and an association with infectious mononucleosis has been found. The challenge is to determine the causes of the EBV negative tumours where various occupational factors, immune function, and medical conditions including immunodeficiency have been suggested [Citation4]. In EUROCARE-4, the 5-year age-standardised relative survival of patients with HL was 80% and for NHL 52% [Citation5]. In the period 1958–1987 survival increased in parallel in the Nordic countries for both these malignancies, although the trend in Icelandic HL survival was steeper [Citation6]. The trend in incidence in all Nordic countries up to 1997 was uniformly increasing for NHL but declining somewhat for HL [Citation7]. The Nordic survival was similar or slightly above the European average [Citation5].
Multiple myeloma comprises just below 1.5% of the total cancer incidence, the manifestation of the disease is variable and it can be difficult to diagnose [Citation8]. The suspected risk factors for multiple myeloma include autoimmune disorders, chronic immune stimulation, ionising radiation, occupational exposures, chemicals, alcohol, and tobacco [Citation8]. The incidence varies considerably internationally and detection depends on the availability and use of diagnostic methods. Within the Nordic countries, access to, and utilisation of diagnostic methods are today similar; however, the variable inclusion of MGUS (monoclonal gammopathy of unknown significance), a precursor to multiple myeloma, in the incidence figures may bring about certain differences between countries. Survival was studied in a Nordic study predicting cancer mortality with survival in Denmark about 10 percentage points below the other Nordic countries up to 1987 [Citation6].
Internationally the leukaemias have been classified differently in different studies. The most recent Cancer Incidence in Five Continents Volume has followed the ICD categories for lymphoid, myeloid and unspecified leukaemia, or as one broad category “leukaemia” [Citation9]. In the EUROCARE study, except for “unspecified”, the ICD categories were further subdivided into acute and chronic, where the acute is dominated by acute myeloid leukaemia (AML) and the chronic by chronic lymphocytic leukaemia (CLL), however, some countries were unable to present sufficient data [Citation5]. The NORDCAN database as it is today only differentiates between “acute leukaemias”, comprising 1% of all cancers, and “other leukaemia” comprising 1.5% [Citation1]. Age is an important factor, given that 20% of all acute leukaemias occur among children (aged 0–14 years at diagnosis), with incidence low up to age 55 and increasing rapidly thereafter. The type that predominates in young age is acute lymphocytic leukaemia (ALL) and is accompanied by acute myeloid leukaemia (AML) among older patients [Citation10]. Other leukaemia is dominated by CLL which, occurs predominantly from ages 55 years and above, and also includes chronic myeloid leukaemia (CML) that in contrast to CLL can be seen in young individuals. Risk factors for leukaemia include both ionising radiation, occupational exposures such as benzene, iatrogenic exposures with radiation and chemotherapy [Citation10].
In this study we follow the survival of Nordic patients diagnosed with HL and NHL, multiple myeloma, and leukaemia, 1964–2003 up until the end of 2006.
Material and methods
We used the NORDCAN database, and hence the data have been checked and converted to well-defined entities, as described by Engholm et al. [Citation11].
We included all cancer patients diagnosed with malignant neoplasms of the lymphoid and haematopoietic tissues (defined as ICD-10 C81-C95) 1964–2003 in Denmark, Finland, Iceland, Norway and Sweden, and supplemented the cancer records with individual records for death up until the end of 2006. The data were analysed according to the individual NORDCAN entities separating the lymphomas into Hodgkin lymphoma (ICD-10 C81) and non-Hodgkin lymphoma (ICD-10 C82-C85, C96), multiple myeloma (ICD-10 C90), and the leukaemias into acute (ICD-10 C91-95 except C9X.0) and other (ICD-10 remaining C91-95). As described in detail [Citation12] we used the cohort survival method for the first seven 5-year periods in each country from 1964–1998, and a hybrid analysis combining period and cohort survival in the last period 1999–2003 [Citation13]. Country-specific life tables were used to calculate the expected survival. Age-standardisation was performed using standard weight distributions (ICSS standards) as in the EUROCARE-4 analysis [Citation14]. Patients were followed until death, emigration or loss to follow-up or to the end of 2006. Excess mortality rates were stratified into short intervals after the diagnosis: the first month, one to three months, 4–12 months and yearly intervals thereafter. We present age-standardised (World) incidence and mortality rates, 5-year relative survival ratios and excess mortality rates for the follow-up periods, the first month, one to three months and two to five years following diagnosis, as well as age-specific 5-year relative survival ratios by country, sex and age.
Results
Hodgkin lymphoma (C81)
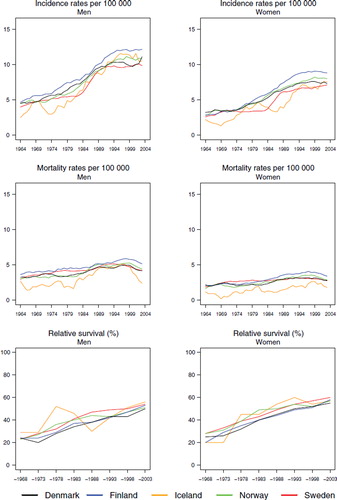
Incidence and mortality. Hodgkin lymphoma is a rare malignancy with an age-standardised incidence of 2 to 3 per 100 000 for both men and women in the Nordic countries. For males, incidence slowly decreased since 1970 when it peaked at around 3 per 100 000 (). There was a corresponding decreasing trend among women until the early-1980s. Incidence rose again thereafter and ended in 1999–2003 at about the same level observed in the mid-1960s. In recent years, incidence has been lowest in Sweden in both sexes and highest in Finland (and Iceland, although based on small numbers). Mortality rates constantly decreased during the period of observation (). Compared to females, the mortality levels in men were slightly higher in all countries, about 0.3 per 100 000 among men and 0.2 among women.
Figure 1. Trends in age-standardised (World) incidence and mortality rates per 100 000 and age-standardised (ICSS) 5-year relative survival for Hodgkin lymphoma by sex and country. Nordic cancer survival study 1964–2003.
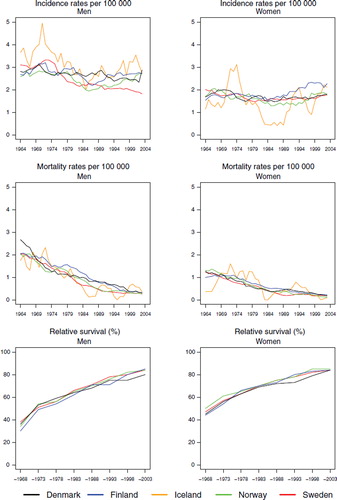
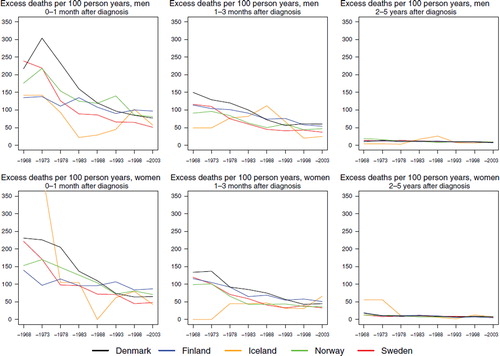
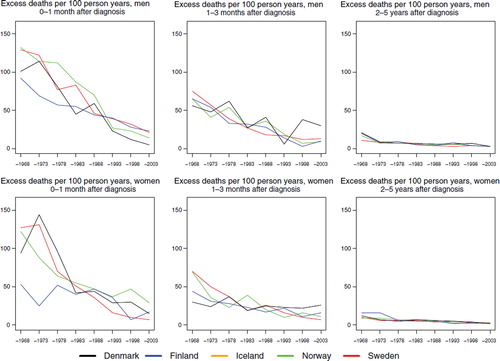
Survival. Age-standardised 5-year relative survival has increased in a similar manner in all Nordic countries and is currently about 85% for both sexes, except for males in Denmark where it is slightly lower (, ). Evaluating survival in terms of excess mortality as a function of time since diagnosis reveals some differences. Excess mortality during the first month after diagnosis was more uneven between countries among women in the early years and was reduced to about 50 per 100 person years by the early-1980s (). Finnish women had a low excess mortality from the 1960s. The reduction of excess death rates among males soon after diagnosis has taken place more gradually, but the excess death rates are currently at about the same level as among women. The excess death rates one to three months after diagnoses have dropped in Norway and Sweden for both sexes and among males in Finland, and to some extent among males in Denmark. No substantial changes with calendar time were seen for Danish and Finnish women one to three months after diagnosis, but within two to five years of follow-up there were few differences between the countries. Iceland was not included in the comparisons as the number of cases was small.
Table I. Trends in survival for Hodgkin lymphoma by sex and country. Number of tumours (N) included and the 5-year age-standardised (ICSS) relative survival in percent with 95% confidence intervals (RS (CI)). Nordic cancer survival study 1964–2003.
Figure 2. Trends in age-standardised (ICSS) excess death rates per 100 person years for Hodgkin lymphoma by sex, country, and time since diagnosis in Nordic cancer survival study 1964–2003. No Icelandic curves. Too few patients to calculate rates for Iceland.
Survival declined with increasing age in all countries. In patients aged 70–89, the 5-year relative survival was below or close to 50% although there were considerable differences between the countries, especially in males (survival in Denmark was 34, Norway 37, Sweden 43, and Finland 53%). Survival has increased over the years in all age groups, most notably in 40–49-year-olds, where relative survival was around 40–50% in the 1960s, but rose to above 90% in all countries in the latest study period ().
Table II. Trends in 5-year age-specific relative survival in percent after Hodgkin lymphoma by sex and country. Nordic cancer survival study 1964–2003.
Non-Hodgkin lymphoma (C82-C85,C96)
Incidence and mortality. The incidence increased in all the Nordic countries () until the 1990s, and in 2003 the age-standardised incidence among men varied between 10 per 100 000 in Sweden to 12.2 in Denmark and for women between 7.1 (Sweden) to 8.8 (Denmark). Mortality has also increased but to a lesser extent than incidence, with a slight decline after year 2000 (). Over the years, but notably from the late-1980s, the gap between incidence and mortality has widened.
Figure 3. Trends in age-standardised (World) incidence and mortality rates per 100 000 and age-standardised (ICSS) 5-year relative survival for non-Hodgkin lymphoma by sex and country. Nordic cancer survival study 1964–2003.
Survival. The 5-year age-standardised relative survival increased from around 20–30% in 1964–1968 to 50–60% in 1999–2003 and was marginally higher in women than in men (; ). The differences between the Nordic countries were small in general, however, with survival highest in Sweden and lowest in Finland and Denmark. Age at diagnosis is an important predictor of the 5-year relative survival in all calendar periods, highest (between 70–80%) for cancers diagnosed in 1999–2003 at ages 0–49 years and falling to 40–50% for ages 70–79. The survival estimates were fairly similar across the Nordic countries ().
Table III. Trends in survival for non-Hodgkin lymphoma by sex and country. Number of tumours (N) included and the 5-year age-standardised (ICSS) relative survival in percent with 95% confidence intervals (RS (CI)). Nordic cancer survival study 1964–2003.
Table IV. Trends in 5-year age-specific relative survival in percent after non-Hodgkin lymphoma by sex and country. Nordic cancer survival study 1964–2003.
The excess death rates by calendar period and time since diagnosis () clearly demonstrate that the inter-country differences, especially in earlier time periods, were largest during the first month of follow-up and largely were eliminated after two years of follow-up. The differences in excess death rates between the Nordic countries have diminished by calendar time and were minor from the late 1980s onwards.
Multiple myeloma (C90)
Incidence and mortality. The incidence increased among men in the Nordic countries reaching a stable rate of 3 per 100 000 in the mid-1980s, except for Norway where the incidence reached 4.3 (). For women, the incidence followed the same pattern and reached a level of 2 to 3 per 100 000 for the period 1999–2003. The mortality rate followed the incidence at a slightly lower level, reaching 2–3 per 100 000 among men and about 1.5 in women while decreasing slightly from the late-1980s ().
Figure 5. Trends in age-standardised (World) incidence and mortality rates per 100 000 and age-standardised (ICSS) 5-year relative survival for multiple myeloma by sex and country. Nordic cancer survival study 1964–2003.
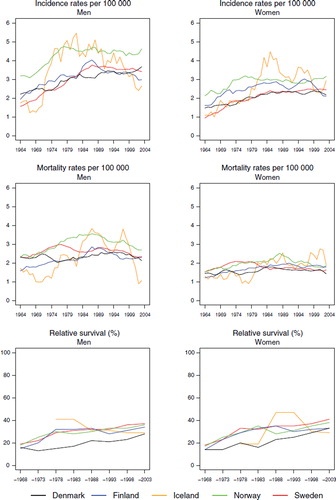
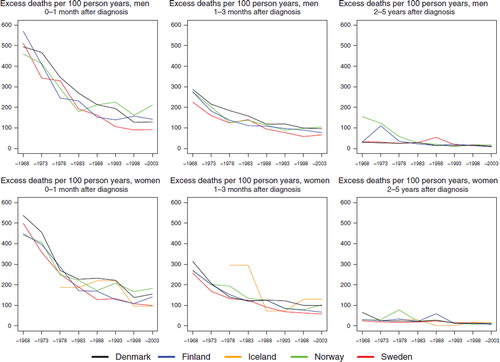
Survival. The 5-year age-standardised relative survival was similar for men and women () and among males increased steadily over the observation period in each country from below 20 to 28% in Denmark, to 29% in Iceland, and to 34, 36, and 37% in Finland, Norway and Sweden, respectively by 1999–2003. In women, the most recent survival estimates ranged from between 29 and 41%. The excess mortality was highest during the first month after diagnosis and in Denmark, although for the most recent time period, the rate of Finland ranked first (). The difference between countries largely disappeared with calendar time, as did the level of the excess mortality during the first month, diminishing to one third of that seen in 1964–1968. The excess death rates in the period one to three months after diagnosis were almost the same in all countries and decreased slightly over time. Two to five years after diagnosis the excess death rates were lower and similar over time and between countries. The survival also depended on age at diagnosis (), with patients diagnosed at younger ages having higher levels of survival than patients diagnosed at older ages. It is noteworthy that, relative to the other countries, Danish patients diagnosed at ages 0–49 in the period 1999–2003 had a rather low survival – between 15–26 percentage points lower in men and 14–19 percentage points lower in women. Sweden and Norway had the highest survival in almost all age groups for patients diagnosed 1999–2003.
Figure 6. Trends in age-standardised (ICSS) excess death rates per 100 person years for multiple myeloma by sex, country, and time since diagnosis in Nordic cancer survival study 1964–2003.
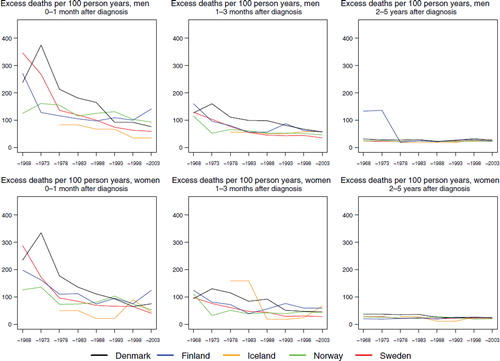
Table V. Trends in survival for multiple myeloma by sex and country. Number of tumours (N) included and the 5-year age-standardised (ICSS) relative survival in percent with 95% confidence intervals (RS (CI)). Nordic cancer survival study 1964–2003.
Table VI. Trends in 5-year age-specific relative survival in percent after multiple myeloma by sex and country. Nordic cancer survival study 1964–2003.
Acute and other leukaemia (C91–95)
Incidence and mortality. The trends in incidence for both acute and other leukaemia were fairly similar in all countries, except Sweden, with rates for acute leukaemia slightly increasing until the mid-1980s to a level of 4 to 5 in men, and between 3 and 4 per 100 000 in women (). The incidence of acute and other leukaemia in Sweden was low compared to the other Nordic countries until the late-1980s, although corresponding mortality was similar across the Nordic populations. The incidence of other leukaemia was a little higher for men ranging between 4 and 6 but somewhat lower for women at between 2 and 3 per 100 000 (). The mortality from both acute and other leukaemia has declined over time, particularly for the latter group. For both cancer forms the mortality levels in the last period have been close to 2 per 100 000 ( and ).
Figure 7. Trends in age-standardised (World) incidence and mortality rates per 100 000 and age-standardised (ICSS) 5-year relative survival for acute leukaemia by sex and country. Nordic cancer survival study 1964–2003.
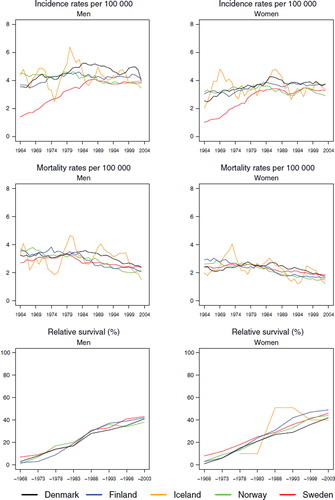
Figure 8. Trends in age-standardised (World) incidence and mortality rates per 100 000 and age-standardised (ICSS) 5-year relative survival for other leukaemia by sex and country. Nordic cancer survival study 1964–2003.
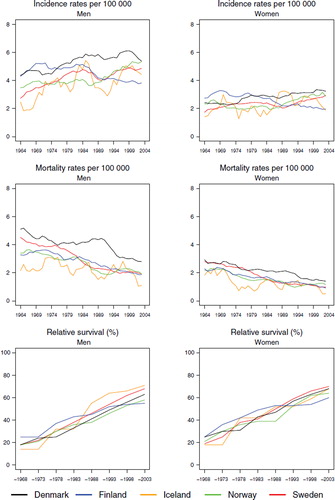
Survival and excess mortality. In agreement with the above trends, 5-year age-standardised relative survival for acute leukaemia increased during the observation period from below 10 to above 40% for both sexes (), as that did for other leukaemia, from below or close to 20% to a level of between 60–70% in the last study period, with little difference observed between the Nordic countries (). It is noteworthy that the survival increases were seen at ages of diagnosis up to 70 years (), with survival estimates between 72–81% in men and women diagnosed below the age of 50 in Denmark, Finland, Norway and Sweden in 1999–2003. For other leukaemia, a similar pattern emerged () and here quite large increases in survival in the older age groups were observed.
Table VII. Trends in survival for acute leukaemia by sex and country. Number of tumours (N) included and the 5-year age-standardised (ICSS) relative survival in percent with 95% confidence intervals (RS (CI)). Nordic cancer survival study 1964–2003.
Table VIII. Trends in survival for other leukaemia by sex and country. Number of tumours (N) included and the 5–year age–standardised (ICSS) relative survival in percent with 95% confidence intervals (RS (CI)). Nordic cancer survival study 1964–2003.
Table IX. Trends in 5–year age–specific relative survival in percent after acute leukaemia by sex and country. Nordic cancer survival study 1964–2003.
Table X. Trends in 5–year age–specific relative survival in percent after other leukaemia by sex and country. Nordic cancer survival study 1964–2003.
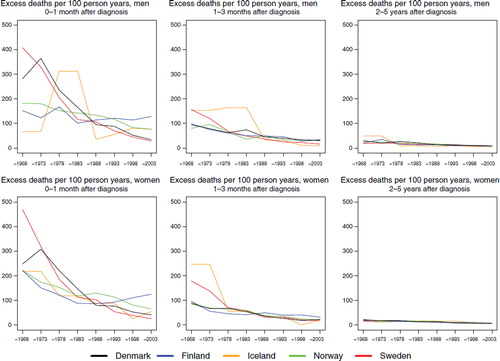
The excess mortality rates for both leukaemia groups ( and ) were highest in the earliest time periods and also within the first month of diagnosis, with a clear decline in excess mortality by calendar year of diagnosis. There were no substantial differences between the countries, and the excess mortality rates one to three months after diagnosis were lower than in the first month, with only minor differences between the countries and over time two to five years after diagnosis.
Discussion
Large randomised international trials and advances in diagnostics and staging procedures have resulted in major improvements in chemotherapeutic regimes with fewer side effects and better average outcomes among patients diagnosed with cancers of the lymphatic and haematopoietic tissues. While individually-tailored treatments may have a more important role in the future, conventional chemotherapy remains the effective and standard mode of treatment at present. HL is an early example of the benefits of large randomised trials, leading to clear improvements in both radiotherapy and chemotherapy, and with treatment for childhood leukaemia paving the way for more effective therapies for other lymphomas and leukaemias. The use of cytotoxic drugs moving from single agents decades ago to combination regimes, coupled with an increasing ability to reduce the impact of side effects and thereby increasing drug tolerance, are clearly reflected in the increasing relative survival and declining mortality over time, contrasting the relatively stable incidence. The EUROCARE-4 study [Citation5] points to survival in Eastern Europe being lower than in other areas, perhaps further supporting the importance of the adoption of new technologies for better diagnostics and financial capabilities to afford new drugs in the treatment of these patients. The reduction of excess mortality during the first months after diagnosis over time also indicates that there is a tendency toward initiating treatment immediately, although clearly there are still some variations in this practice.
Patients in Denmark have a higher excess mortality one to three months after diagnosis than their Nordic counterparts, which may indicate later diagnoses leading to less favourable effects of treatment, or co-morbidity resulting in death from competing causes. Supportive care during the first months after diagnosis also plays a role in patient survival, but we have no reason to suspect that the quality differs materially between the Nordic countries. A minor part of the earlier increases in HL survival might be attributed to artefact in that certain NHL cases associated with poorer prognosis may have been previously misclassified as HL, but with advances in diagnostics, such cases have become increasingly more likely to be correctly classified as NHL [Citation15].
The survival of NHL patients is rather homogenous in the Nordic populations and close to, or above the European average [Citation5], although substantially lower (30 percentage points) than the survival observed among HL patients. The lack of variation is in contrast to the European picture of heterogeneity and a clear east-west gradient, with the poorest survival seen in several Eastern European populations [Citation5]. The interpretation of the definition of NHL and the treatment options likely differ by European region, but it is probable that definitions and the quality of treatment and care within the Nordic countries are fairly similar, especially after the formation of the Nordic Lymphoma Group (www.nordic-lymphoma.org). The only differences we have seen are an overrepresentation of elderly patients with NHL in Sweden and a somewhat younger distribution of patients in Iceland [Citation1], although neither appears to have impacted on the age-standardised survival rates. Also during the study period, we observed the effect of changes in classification, most notably in Sweden, leading to an incidence level in 2003 not dissimilar to 1989. This is likely a consequence of new coding rules separating more precisely chronic lymphocytic leukaemias and lymphomas. That there has been a slight downturn in mortality in the last decade, coupled with a steady increase in relative survival to a level similar across countries, points to the likelihood of concurrent adoption of new therapies as a result of international collaborations in haematology such as the CALGB (Cancer and Leukaemia Group B) initiated in the USA in the mid-1950s, and in the Nordic countries the national and the Nordic Lymphoma Group established from the 1980s. The observed pattern of a high excess rate of deaths during the first three months after diagnosis from the 1960s to the 1980s and a levelling off thereafter are also indicative of an effect of the improvement of chemotherapy and radiotherapy and a widespread use of these treatment modalities.
Assuming that the incidence of multiple myeloma is defined in the same way in all of the Nordic countries, it is of note that survival appears to differ between the Nordic countries. Only in Sweden, Finland and Norway has survival reached the reported European average in EUROCARE-4 [Citation5], although it is apparent that the definition of multiple myeloma and diagnostics varies across Europe. The excess mortality during the first month after diagnosis and the somewhat poorer survival among younger patients in Denmark may reflect Denmark’s position as the country with the poorest survival among the Nordic countries. Since treatment is considered to be similar in the Nordic countries [Citation16,Citation17], and a Nordic collaboration (NMSG – Nordic Myelomatosis Study Group) dedicated to multiple myeloma and MGUS has been studying differences in incidence and survival without any clear cut explanations as yet, co-morbidity could be a candidate as an explanatory factor.
The Nordic countries are rather similar with respect to rates of leukaemia incidence, as are trends in incidence, mortality and survival for both acute and other leukaemias. The only exception is Sweden where the underreporting of leukaemia and lymphoma incidence is evident until the 1980s [Citation18] and still present in 1998 [Citation19], and clearly indicated by the higher mortality than incidence rates during this period. The reason for the underreporting is largely unknown but may be due to the fact that leukaemias are often diagnosed at haematology departments without the use of services from pathology departments. Reporting from pathology labs are traditionally well integrated with cancer registration however reported to be less so in Sweden [Citation19]. The problems in reporting is to some extent counteracted in the other Nordic countries by supplementary use of information from death certificates which is not the case in Sweden. It is worthy of note that survival after an acute leukaemia is similar in each Nordic population, even in the period where registration is considered incomplete in Sweden. We see the same pattern with respect to excess mortality for other cancer sites, reflecting that the time window shortly after diagnosis is very critical to prognosis. The increase in survival for all types of leukaemia can likely be ascribed to improved therapy, with chemotherapy the modality of choice. Evidently the crude division into acute and other leukaemia is insufficient for clinical purposes, and our results capture only the general observation of rapid and successful developments in treatment. The survival results we observe for acute leukaemia among younger patients predominantly reflect acute lymphocytic leukaemia (ALL) survival and among older patients those diagnosed with AML. For the other leukaemias, survival among the older age groups is dominated by CLL. It is well known that treatment regimes differ between the leukaemia subtypes, but for all, irrespective of the age of the patient, the focus is on the control of the systemic disease. More recent successful treatment regimes e.g. for chronic myeloid leukaemia (CML), where imatinib (Gleevec) is the current treatment of choice [Citation20], offer the promise of further improvement in leukaemia survival. The latter is believed to explain a marked increase in the median survival of CML, an observation that is unlikely to be picked up in a future study given that CLL dominates the other leukaemia group, but could be studied using the sub-typing of leukaemias usually available today in the cancer registries [Citation20]. The classifications in the past of lymphomas and leukaemias alongside the changes in diagnostic precision will influence the survival trends, and a proper analysis on subtypes should be combined with quality control and a complete revision of data and the pathology for the entire time period under study.
In conclusion, the lymphoid and haematopoietic cancer incidence, mortality and survival in the Nordic countries are much more similar than is seen for many other cancer forms. This may be expected given the nature of the diseases and that the first-line treatment is often systemic. We do however observe a general pattern that Danish patients with these diseases fare a little worse than their Nordic neighbours. The excess mortality rates for Danish patients one to three months after diagnosis rank highest, and role of co-morbidity related to lifestyle factors and its contribution to the success of chemotherapy and radiation should be considered in further studies.
Acknowledgements
The Nordic Cancer Union (NCU) has financially supported the development of the NORDCAN database and program, as well as the survival analyses in this project.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Engholm G, Ferlay J, Christensen N, Bray F, Gjerstorff ML, Klint Å, . NORDCAN: Cancer incidence, mortality and prevalence in the Nordic countries, Version 3.4. Association of Nordic Cancer Registries. Danish Cancer Society. 2009 Available from http://www.ancr.nu.
- Hartge P, Wang SS, Bracci PM, Devesa SS, Holly EA. Non- Hodgkin lymphoma. Schottenfeld D, Fraumeni JF Jr. Cancer epidemiology and prevention. 3rd. New York: Oxford University Press; 2006. 898–918.
- Ekström-Smedby K. Epidemiology and etiology of non-Hodgkin lymphoma – a review. Acta Oncol 2006;45:258–71.
- Mueller NE, Grufferman S. Hodgkin lymphoma. Schottenfeld D, Fraumeni JF Jr. Cancer epidemiology and prevention. 3rd. New York: Oxford University Press; 2006. 872–97.
- Sant M, Allemani C, Santaquilani M, Knijn A, Marchesi F, Capocaccia R; the EUROCARE Working Group. EUROCARE-4. Survival of cancer patients diagnosed in 1995-1999. Results and commentary. Eur J Cancer 2009;45: 931–91.
- Engeland A, Haldorsen T, Tretli S, Hakulinen T, Hörte LG, Luostarinen T, . Prediction of cancer mortality in the Nordic countries up to the years 2000 and 2010, on the basis of relative survival analysis. A collaborative study of the five Nordic Cancer Registries. APMIS Suppl. 1995;49:1–161.
- Møller B, Fekjaer H, Hakulinen T, Tryggvadóttir L, Storm HH, Talbäck M . Prediction of cancer incidence in the Nordic countries up to the year 2020. Eur J Cancer Prev 2002;11(Suppl 1):S1–S96.
- Roos AJ, Baris D, Weiss NS, Herrington L. Multiple myeloma. Schottenfeld D, Fraumeni JF Jr. Cancer epidemiology and prevention. 3rd. New York: Oxford University Press; 2006. 919–45.
- Curado MP, Edwards B, Shin HR, Storm H, Ferlay J, Heanue M, . Cancer incidence in five continents. IX, Lyon, France: IARC Scientific Publications 160; 2007.
- Linet M, Devesa SS, Morgan GJ. The leukemias. Schottenfeld D, Fraumeni JF Jr. Cancer epidemiology and prevention 3rd. New York: Oxford University Press; 2006. 841–71.
- Engholm G, Storm HH, Ferlay J, Christensen N, Bray F, Ólafsdóttir E, . NORDCAN: Cancer incidence and mortality in the Nordic countries Acta Oncol 2010.
- Engholm G, Gislum M, Bray F, Hakulinen T. Trends in the survival of patients diagnosed with cancer in the Nordic countries 1964–2003 followed up the end of 2006. Material and methods. Acta Oncol; 2010.
- Brenner H, Rachet B. Hybrid analysis for up-to-date long-term survival rates in cancer registries with delayed recording of incident cases. Eur J Cancer 2004;40:2494–501.
- Corazziari I, Quinn M, Capocaccia R. Standard cancer patient population for age standardising survival ratios. Eur J Cancer 2004;40:2307–16.
- Talbäck M, Rosén M, Stenbeck M, Dickman PW. Cancer patient survival in Sweden at the beginning of the third millennium—predictions using period analysis. Cancer Causes Control 2004;15:967–76.
- Nordic Myelomatosis Study Group. Nordic reference programme on Myelomaosis diagnosis and treatment 2001. Cited 30.11.2009. Available from www.nordic-myeloma.org/content/dk/pdf/referenceprogrammer/referenceprogram_2001.pdf.
- Smith A, Wisloff F, Samson D. Guide on the diagnostics and management of multiple myeloma 2005. Br J Haematol 2005;132:410–51.
- Lund EM, Clemmensen IH, Storm HH, Engholm G. Survey of the Nordic Cancer Registries, Danish Cancer Society, Copenhagen 2000. Available from http://www.ncu.nu/ancr/pdf/survey.pdf 29-08-2009.
- Barlow L, Westergren K, Holmberg L, Talbäck M. The completeness of the Swedish Cancer Register: a sample survey for year 1998. Acta Oncol 2009;48:27–33.
- Thygesen LC, Nielsen OJ, Johansen C. Trends in adult leukaemia incidence and survival in Denmark, 1943–2003. Cancer Causes Control 2009;20:1671–80.