Abstract
Background. Treatment of patients with carcinoma of unknown primary site (CUP) remains a challenge, and no effective second-line treatment has been identified. In CUP patients who are non-responsive or relapse early after first-line platinum/taxane-based regimens, it is likely that gastrointestinal (GI) tract tumours may be overrepresented. These patients could be candidates for GI tract-directed therapy. We here report the results obtained with oxaliplatin and capecitabine as second-line therapy in 25 recurrent/refractory CUP patients following first-line treatment with paclitaxel, cisplatin and gemcitabine. Patients and methods. Patients received capecitabine orally (1000 mg/m2) twice daily, days 1–14, and oxaliplatin (130 mg/m2) intravenously on day 1 in a three-week schedule. Results. Twenty-five CUP patients received a median of three cycles of capecitabine and oxaliplatin as second-line treatment. Histopathological assessments suggested the primary site to be of GI tract origin in the majority of the patients (76%). We found an objective response rate of 13%, a median progression-free survival and overall survival rate of 2.3 and 3.9 months, respectively, and 32% of patients alive at one year after initiation of second-line therapy. The regimen was well tolerated by most patients. Conclusions. This study, demonstrates that there is still a significant need for improved second-line therapy in CUP patients.
Carcinoma of unknown primary site (CUP) accounts for 3–5% of all cancer diagnoses and is characterised by early dissemination, uncommon metastatic sites, and usually a poor prognosis [Citation1,Citation2]. The majority of CUP patients have adenocarcinomas or poorly differentiated carcinomas.
Treatment of the unfavourable subset, i.e. the majority, of CUP patients remains a challenge, and an optimal first-line treatment is still not identified. Regimens with platinum/taxane result in some of the highest response rates and longest median overall survival [Citation3]. However, these regimens are not optimal if the primary tumour is located in the gastrointestinal (GI) tract. In these cases, agents like 5-fluorouracil, oxaliplatin, irinotecan and gemcitabine are recommended. GI-tract regimens have been evaluated in the first-line setting in CUP patients, with lower response rates (mean 22%) and median OS (around 7 months) compared to platinum/taxane containing regimens [Citation4]. Recently, Schuette et al. reported similar results in a phase II trial with oxaliplatin and capecitabine in chemo-naïve CUP patients [Citation5]. Thus, in the unfavourable CUP subset, platinum/taxane-based regimens should still be considered as first-line therapy.
Only a few second-line trials have been conducted in CUP patients [Citation6–9], all with poor outcome. In CUP patients who do not respond or relapse early after first-line platinum/taxane-based regimens it is likely that GI tract tumours may be overrepresented. These patients could be candidates for GI tract-directed therapy.
In the present report we describe the results obtained with oxaliplatin and capecitabine as second-line therapy in 25 CUP patients following first-line treatment with a platinum/taxane-based regimen.
Patients and methods
Patients
Patients were considered to have CUP when diagnostic work-up as recommended by European Society of Medical Oncology (ESMO) failed to identify the primary site of origin [Citation10]. CUP patients belonging to the favourable subsets were excluded.
Since 2003, approximately 120 CUP have been treated with paclitaxel, gemcitabine and platinum as first-line therapy in our department. Forty patients were offered second-line therapy and 25 of these patients were offered oxaliplatin and capecitabine as second-line treatment as described in the present study. All patient files were reviewed for patient characteristics, response, progression to first-line and second-line therapy, treatment-related toxicity and date of death.
Treatment
Patients received capecitabine orally (1000 mg/m2) twice daily, days 1–14, and oxaliplatin (130 mg/m2) intravenously on day 1 of each cycle. Treatment was repeated every 21 days until disease progression, unacceptable toxicity or withdrawal by the patient.
Toxicities were assessed every three weeks. Capecitabine administration was discontinued in cases of grade 2 non-hematological toxicity and continued again when toxicity became grade 1 (Common Terminology Criteria for Adverse Events (CTCAE) v 3.0). Capecitabine was reduced by 25% in cases of grade 3 non-hematological toxicity or grade 4 haematological toxicity. In persisting pain-related neuropathy, oxaliplatin dosage was reduced by 25%.
Assessment/evaluations
The primary endpoint was progression-free survival (PFS). Secondary endpoints were response rate, toxicity and overall survival (OS). Radiological tumour responses were evaluated according to RECIST (1.0) guidelines [Citation11]. The objective response rate was calculated on intent-to-treat basis among all patients with target lesions/measurable disease who initiated treatment. PFS was calculated from the first day of second-line treatment to the first sign of progressive disease (PD), last date of follow-up or death. OS was calculated from the date of initiation of second-line treatment as well as from initiation of first-line treatment until the date of death or last follow-up.
All data concerning PFS and OS were analysed according to the Kaplan-Meier method by using the SPSS statistical software package (version 15).
Toxicity was evaluated according to CTCAE v 3.0.
Results
Patient characteristics
Patient characteristics are shown in . Twelve of the 25 patients (48%) had achieved an objective response to first-line therapy (paclitaxel, cisplatin and gemcitabine) with a median PFS of 6.2 months (95% CI 5.2–7.3 months).
Table I. Patient characteristics (second-line therapy – capecitabine and oxaliplatin).
Based on histopathological assessment a primary tumour was suggested to originate from the GI tract in the majority of patients (76%). Thirteen of the 25 patients (52%) presented with more than two metastatic sites () and lymph nodes were the most frequent disease localisation (68%), followed by liver (52%), lung (32%), bones (20%) and peritoneal involvement (16%). Elevated lactate dehydrogenase (LDH) levels at baseline were found in 15 patients (60%).
Twenty-three patients had measurable lesions. Two patients had only peritoneal involvement and therefore had only non-target lesions.
Treatment
A total of 99 treatment cycles were administered with a median of three cycles per patient (range: 1–19). An initial dose reduction of 25% of oxaliplatin, capecitabine or both was necessary in five patients. Seven patients discontinued treatment prior to response evaluation due to clinical evidence of early disease progression (n = 4), treatment-related death (febrile neutropenia) (n = 1), a pulmonary embolus (n = 1) and congestive heart failure (n = 1), respectively.
Efficacy
Twenty-three patients had measurable lesions and were included in the response assessment. On intent-to-treat analysis, a partial response was observed in three of the 23 patients (13%; 95% CI 4.2–33.5%) (). The responses were observed in lymph nodes (one patient) and liver (two patients). The two patients with liver involvement also obtained a partial response with first-line treatment.
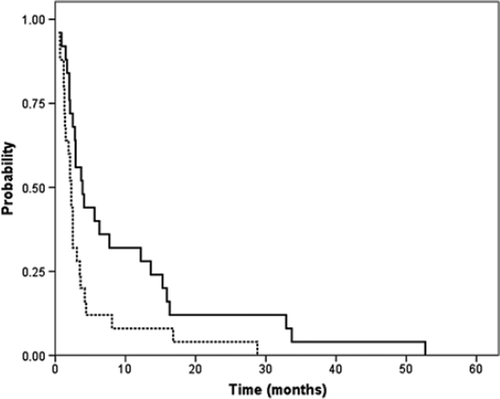
All enrolled patients were analysed for progression-free survival (PFS) and overall survival (OS). illustrates the PFS and OS curves for all 25 patients. The median PFS duration was 2.3 months (95% CI 1.8–2.8 months) with a 1-year PFS rate of 8% (two patients). The median survival duration was 3.9 months (95% CI 1.9–5.9), with a 1-year survival rate of 32% (8 patients) and 2-year survival rate of 12% (3 patients). The median OS from initiation of first-line treatment to death for the 25 patients was 15.4 months (95% CI 8.2–22.5 months).
Figure 1. Kaplan-Meier curves for progression-free survival (broken line) and overall survival (continous line) (n = 25). Median PFS was 2.3 months (95% CI: 1.8–2.8) and median OS was 3.9 months (95% CI: 1.9–5.9).
At progression on second-line therapy a primary tumour was identified in three patients (renal pelvis, the kidney and the appendix (goblet cell carcinoid)).
Toxicity
Treatment-related toxicities are listed in . Grade 3/4 toxicities were uncommon: fatigue (4%), thrombocytopenia (4%) and nausea and vomiting (2%). The dosage of oxaliplatin was reduced by 25% in six patients (24%) due to pain-related neuropathy. Oxaliplatin was discontinued in one patient due to grade 3 neuropathy. Capecitabine was reduced in three patients because of grade 3 nausea/vomiting or diarrhoea.
Table II. Treatment-related toxicities* (No of cycles = 99) - rates in brackets.
Discussion
Effective second-line therapy for CUP patients has not yet been identified. Only four prospective trials of second-line therapy have been conducted in CUP patients since 2001 (). In total, 119 patients were enrolled in these trials using either single-agents or combination regimens. They all produced low response rates and the median survival time was short (range 3–8 months).
Table III. Second-line chemotherapy regimens in CUP patients.
Gene expression profiling and post-mortem examinations indicate that only 35–45% of the primary tumours in CUP patients are of GI origin (recently reviewed by Pentheroudakis et al.) [Citation4]. In agreement with these observations, first-line taxane/platinum-based regimens offer considerably better response rates, PFS and OS compared with GI tract-directed regimens [Citation3,Citation4,Citation12]. In CUP patients who are non-responsive or relapse after platinum/taxane-containing regimens, primary GI tract tumours may be overrepresented. Thus, these patients could be candidates for GI-tract-directed therapy. In line with this, most CUP patients in our department have since 2003 been offered oxaliplatin and capecitabine as second-line therapy following first-line therapy with a combination of paclitaxel, gemcitabine and platinum [Citation12].
We here report the results obtained with the above second-line regimen in 25 recurrent/refractory CUP patients. In summary, we found an objective response rate of 13%, a median progression-free survival and overall survival rate of 2.3 and 3.9 months, respectively, and eight patients (32%) were alive one year after initiation of second-line therapy. The treatment-related toxicities were mild to moderate in the majority of patients.
A relatively high one-year survival rate was observed in the present study. The reasons for this could be 1) all eight patients were offered further treatment at progression on capecitabine and oxaliplatin (); 2) in the follow-up period three of these patients had a primary tumour identified (kidney, renal pelvis and appendix (goblet cell carcinoid)), and they were consequently offered site-specific treatment; 3) the remaining five CUP patients all had involvement of only one metastatic site (), which is generally considered a good prognostic factor [Citation13,Citation14]. Two patients had liver involvement, and both obtained a partial response to capecitabine and oxaliplatin. Three patients had lymph node involvement; 4) patient selection bias may also be considered.
Despite the relatively high one-year survival rate, our study shows that there is still a significant need for improved second-line therapy in CUP patients. In particular, prospective trials combining conventional chemotherapy with targeted biological agents are warranted. Hainsworth et al. [Citation15] have shown that the combination of bevacizumab/erlotinib has a substantial activity in the second- and third-line setting for CUP patients, producing a response rate of 10% and median PFS and OS rate of 3.9 months and 7.4 months, respectively.
Therefore, targeted therapy with a GI tract-directed regimen might further improve the outcome in CUP patients who have relapsed or not responded to first-line therapy.
Acknowledgements
We would like to thank Kell Osterlind for statistical assistance.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Pavlidis N, Briasoulis E, Hainsworth J, Greco FA. Diagnostic and therapeutic management of cancer of an unknown primary. Eur J Cancer 2003;39:1990–2005.
- Pavlidis N, Fizazi K. Carcinoma of unknown primary (CUP). Crit Rev Oncol Hematol 2009;69:271–8.
- Pavlidis N. Forty years experience of treating cancer of unknown primary. Acta Oncol 2007;46:592–601.
- Pentheroudakis G, Greco FA, Pavlidis N. Molecular assignment of tissue of origin in cancer of unknown primary may not predict response to therapy or outcome: A ystematic literature review. Cancer Treat Rev 2009;35:221–7.
- Schuette K, Folprecht G, Kretzschmar A, Link H, Koehne CH, Gruenwald V, . Phase II trial of capecitabine and oxaliplatin in patients with adeno- and undifferentiated carcinoma of unknown primary. Onkologie 2009;32:162–6.
- Culine S, Ychou M, Fabbro M, Romieu G, Cupissol D. 5-fluorouracil and leucovorin as second-line chemotherapy in carcinomas of unknown primary site. Anticancer Res 2001;21:1455–7.
- Hainsworth JD, Burris HA, III, Calvert SW, Willcutt NT, Scullin DC, Jr., Bramham J, . Gemcitabine in the second-line therapy of patients with carcinoma of unknown primary site: A phase II trial of the Minnie Pearl Cancer Research Network. Cancer Invest 2001;19:335–9.
- Hainsworth JD, Spigel DR, Raefsky EL, Kuzur ME, Yost K, Kommor M, . Combination chemotherapy with gemcitabine and irinotecan in patients with previously treated carcinoma of an unknown primary site: A Minnie Pearl Cancer Research Network Phase II trial. Cancer 2005;104:1992–7.
- Pouessel D, Culine S, Becht C, Romieu G, Fabbro M, Ychou M, . Gemcitabine and docetaxel after failure of cisplatin-based chemotherapy in patients with carcinoma of unknown primary site. Anticancer Res 2003;23:2801–4.
- Briasoulis E, Pavlidis N, Felip E. Cancers of unknown primary site: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol 2009;20(Suppl 4):154–5.
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, . New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205–16.
- Møller A, Pedersen K, Gothelf A, Daugaard G. Paclitaxel, cisplatin and gemcitabine in treatment of carcinomas of unknown primary site, a phase II study. Acta Oncol 2010;49:423–30.
- Culine S, Kramar A, Saghatchian M, Bugat R, Lesimple T, Lortholary A, . Development and validation of a prognostic model to predict the length of survival in patients with carcinomas of an unknown primary site. J Clin Oncol 2002;20:4679–83.
- Ponce LJ, Segura HA, Diaz BR, Gimenez OA, Aparisi AF, Fleitas KT, . Carcinoma of unknown primary site: Development in a single institution of a prognostic model based on clinical and serum variables. Clin Transl Oncol 2007;9:452–8.
- Hainsworth JD, Spigel DR, Farley C, Thompson DS, Shipley DL, Greco FA. Phase II trial of bevacizumab and erlotinib in carcinomas of unknown primary site: The Minnie Pearl Cancer Research Network. J Clin Oncol 2007;25:1747–52.
