To the Editor
Approval of the tyrosine kinase inhibitor (TKI) imatinib in 2001 provided an effective treatment for chronic myeloid leukaemia (CML). CML is a myeloproliferative disorder of pluripotent haematopoietic stem cells associated with the oncogenic fusion gene BCR-ABL, coding for a protein with tyrosin kinase activity [Citation1]. Reported intolerance and/or resistance to imatinib prompted the development of the second-generation TKIs nilotinib and dasatinib [Citation2]. Due to their similarities regarding chemical structure and pharmacologic activity [Citation3], reported cardiotoxicity for imatinib [Citation4] may also be associated with the other TKIs.
We report on a patient with CML and a pre-existing coronary heart disease (diagnosed after death) who developed a fatal myocardial infarction (MI) while being treated with nilotinib.
Case report
A 60-year-old male patient with CML (diagnosed 1997; in remission/chronic phase), successfully treated with imatinib for six years, was switched to nilotinib 400 mg twice daily due to imatinib intolerance (diarrhoea, eyelid oedema) and suspected resistance. Four months later, the patient complained about retrosternal pressure sensations associated with chills, fever, weakness, and anxiety, and suffered since then from dyspnoea at exertion and from episodes of retrosternal pressure mainly at night. In a cardiological examination one month later echocardiography was normal according to age (diastolic abnormal relaxation grade II) and ergometry (exercise-tolerance, Ramp protocol) revealed a slightly reduced physical performance due to lack of training. Importantly, it did not reveal any signs of coronary heart disease, but a coronary angiography was not performed. Two months later, the patient was hospitalised due to angina pectoris-like symptoms. Concomitant medications were escitalopram in the morning and torasemide if needed. Again, there were no clinical or electrocardiographic signs of coronary heart disease, and a second ergometry was also negative. Liver values, electrolytes and creatinine were normal. The patient and his family were convinced, however, that nilotinib was the causative agent, and the patient stopped the treatment. Treatment with acetylsalicylic acid 100 mg was started. Two weeks later , nilotinib was restarted at a dosage of 600 mg per day. Serum biochemistry at that time did not reveal any particularities. One month later, the patient had to be resuscitated due to a cardiac arrest caused by ventricular fibrillation. After stabilisation, the electrocardiogram (ECG) yielded a loss of the R wave across the entire front wall, and troponin and creatine kinase levels were markedly elevated (208 μg/L [Reference: 0.00–0.80 μg/L] and 11 000 U/L [Reference: 24–190 U/L], respectively). The patient died on day 3 of hospitalisation due to multi-organ failure.
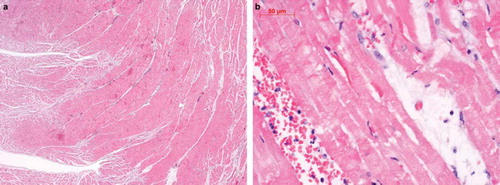
Autopsy showed a recent infarction of the entire interior myocardium of the left ventricle ( and ), obliterating coronary sclerosis of the ramus interventricularis anterior and circumflexus, myocardial hypertrophy of the left ventricle, atherosclerosis of the infrarenal aorta, and acute bronchopneumonia.
Figure 1a,b. Hematoxylin-eosin-stained sections of the left ventricle obtained at autopsy. shows a recent infarction of the interior ventricular myocardium (10×), shows a section of endocardial region with an acute infarction of the subendocardial myocardium at higher power (40×).
Apart from dyslipidaemia (including reported sleep apnoea at start of Nilotinib treatment) and increased body mass index (27 kg/m2), the patient did not have any comorbidities or cardiac risk factors (such as diabetes, hypertension, or smoking).
The case was reported to the national Pharmacovigilance Center Swissmedic.
Discussion
Cardiotoxicity has only recently been reported for drugs that target tyrosine kinases [Citation4,Citation5], but usually not for drugs that inhibit the tyrosine kinase associated with the epidermal growth factor receptor. Hence, cardiotoxicity does not seem to be a class effect of TKIs [Citation6], and may depend on an interaction with certain target receptors. The exact incidence of this adverse effect is unclear [Citation7].
Nilotinib inhibits the activity of the tyrosine kinase BCR-ABL through competitive inhibition of the binding site for ATP with a higher binding affinity and selectivity than imatinib [Citation8]. Further receptors targeted by nilotinib and imatinib include ARG, KIT, PDGFR, DDR1, and NQO2 [Citation3]. The maximum tolerated dose evaluated for nilotinib did not show any dose-limiting cardiovascular effects [Citation8]. Due to observed prolongation of the QT interval and few cases of sudden death [Citation8–10], the drug carries a black box warning from the Food and Drug Administration. Pericardial and pleural effusion, pulmonary oedema, left ventricular dysfunction, atrial fibrillation as well as death due to MI, coronary artery disease, and/or heart failure have rarely been reported in clinical trials with nilotinib [Citation8–10].
Different mechanisms of cardiotoxicity have been described for TKIs depending on their target receptor profile [Citation6]. For imatinib, cardiotoxicity is supposed to be due to mitochondrial damage to cardiomyocytes mediated via the target receptor C-Abl [Citation4]. However, nilotinib and imatinib also target KIT or CD117 [Citation3,Citation6], a receptor for cytokine stem cell factor with tyrosine kinase activity located on haemangioblasts, which are precursors for haematopoietic stem cells and endothelial progenitor cells [Citation6,Citation11]. KIT activation is, amongst others, supposed to be necessary for endothelial progenitor cells to migrate to injured tissue, e.g. after MI [Citation6].
Already at the beginning of treatment with nilotinib, the patient reported here most probably had pre-existing coronary heart disease which was only detected in the autopsy and which was likely the primary cause of the patient's MI. A negative effect of nilotinib can nonetheless not be excluded in this patient considering the temporal association between nilotinib ingestion and the rapid course of coronary artery disease leading to acute MI. Although the patient had suggestive symptoms, coronary artery disease could not be demonstrated before he suffered an acute MI. The patient was investigated using electrocardiography and ergometry, but not by coronary angiography. Sensitivity of ergometry showed a wide variability in meta-analyses (sensitivity 68% [range 23–100%]) [Citation12]. However, patients admitted to the hospital with chest pain without confirmed acute MI and discharged without ECG abnormalities belonged to the low risk group to develop cardiac events or death up to seven years after hospitalisation [Citation13,Citation14]. Abnormalities in the ECG at rest or during exercise increased the risk of a cardiac event by a factor of 11.8 [Citation15]. Normal echocardiography further indicated a good cardiac prognosis in such patients [Citation16]. Hence, an association between nilotinib and the acute occurrence of fatal MI should be considered. A negative influence of the drug on reparatory mechanisms for pre-existing atherosclerotic changes in coronary arteries via inhibition of KIT can be hypothesised.
In conclusion, although cardiotoxicity of nilotinib is supposed to be via mitochondrial damage, a negative effect of the substance on pre-existing atherosclerotic changes or ischaemic processes may also be possible and merits further investigation. If confirmed, coronary angiography in patients ingesting nilotinib and presenting with angina pectoris-like symptoms may be considered. This report emphasises the importance of cardiac monitoring of patients under TKI treatment [Citation7] and of evaluating cardiotoxicity for each TKI individually [Citation6] in order to identify potential molecular targets and risk factors for this adverse event.
Declaration of interest and disclosure: The authors report no conflicts of interest. The work was not sponsored. The authors alone are responsible for the content and writing of the paper. AG has received research support from Bristol Myers Squibb and Novartis. He has served as advisor for Novartis.
References
- Garcia-Manero G, Faderl S, O’Brien S, Cortes J, Talpaz M, Kantarjian HM. Chronic myelogenous leukemia: A review and update of therapeutic strategies. Cancer 2003;98: 437–57.
- Marshall HM, Hammond JM. Treatment options in imatinib-resistant chronic myelogenous leukemia. Ann Pharmacother 2008;42:259–64.
- Hantschel O, Rix U, Superti-Furga G. Target spectrum of the BCR-ABL inhibitors imatinib, nilotinib and dasatinib. Leuk Lymphoma 2008;49:615–9.
- Kerkela R, Grazette L, Yacobi R, Iliescu C, Patten R, Beahm C, . Cardiotoxicity of the cancer therapeutic agent imatinib mesylate. Nat Med 2006;12:908–16.
- Chu TF, Rupnick MA, Kerkela R, Dallabrida SM, Zurakowski D, Nguyen L, . Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet 2007;370:2011–9.
- Force T, Krause DS, Van Etten RA. Molecular mechanisms of cardiotoxicity of tyrosine kinase inhibition. Nat Rev Cancer 2007;7:332–44.
- Orphanos GS, Ioannidis GN, Ardavanis AG. Cardiotoxicity induced by tyrosine kinase inhibitors. Acta Oncol 2009;48: 964–70.
- Kantarjian H, Giles F, Wunderle L, Bhalla K, O’Brien S, Wassmann B, . Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. N Engl J Med 2006; 354:2542–51.
- Kantarjian HM, Giles F, Gattermann N, Bhalla K, Alimena G, Palandri F, . Nilotinib (formerly AMN107), a highly selective BCR-ABL tyrosine kinase inhibitor, is effective in patients with Philadelphia chromosome-positive chronic myelogenous leukemia in chronic phase following imatinib resistance and intolerance. Blood 2007;110:3540–6.
- le Coutre P, Ottmann OG, Giles F, Kim DW, Cortes J, Gattermann N, . Nilotinib (formerly AMN107), a highly selective BCR-ABL tyrosine kinase inhibitor, is active in patients with imatinib-resistant or -intolerant accelerated-phase chronic myelogenous leukemia. Blood 2008;111:1834–9.
- Edling CE, Hallberg B. c-Kit–a hematopoietic cell essential receptor tyrosine kinase. Int J Biochem Cell Biol 2007;39: 1995–8.
- Gibbons RJ, Balady GJ, Bricker JT, Chaitman BR, Fletcher GF, Froelicher VF, . ACC/AHA 2002 guideline update for exercise testing: Summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). J Am Coll Cardiol 2002; 40:1531–40.
- Madsen JK, Hommel E, Hansen JF. Prognostic value of an electrocardiogram at rest and exercise test in patients admitted with suspected acute myocardial infarction, in whom the diagnosis is not confirmed. Eur Heart J 1987; 8:717–24.
- Fruergaard P, Launbjerg J, Jacobsen HL, Madsen JK. Seven-year prognostic value of the electrocardiogram at rest and an exercise test in patients admitted for, but without, confirmed myocardial infarction. Eur Heart J 1993;14:499–504.
- Madsen JK, Thomsen BL, Mellemgaard K, Hansen JF. Independent prognostic risk factors for patients referred because of suspected acute myocardial infarction without confirmed diagnosis. Prognosis after discharge in relation to medical history and non-invasive investigations. Eur Heart J 1988; 9:610–8.
- Muscholl MW, Oswald M, Mayer C, von Scheidt W. Prognostic value of 2D echocardiography in patients presenting with acute chest pain and non-diagnostic ECG for ST-elevation myocardial infarction. Int J Cardiol 2002;84:217–25.
