Abstract
The use of protons for curative treatment of prostate cancer is increasing, either as a single treatment modality or in combination with conventional radiotherapy. The proximity between prostate (target) and rectum (organ at risk) often leads to a compromise between dose to target and organ at risk. Material and methods. The present study describes a method where the distance between prostate and rectum is increased by retraction of the rectum in dorsal direction. Comparative treatment plans with and without retraction of the rectum in the same patients have been studied. Nine patients with biopsy proven, localised adenocarcinoma of the prostate were studied. A cylindrical rod of Perspex was inserted into the rectum. This device allows the rectum to be retracted posteriorly. The patients were given a proton boost of 20 Gy in four fractions of 5 Gy in addition to a conventional photon beam treatment to a dose of 50 Gy in 25 fractions of 2 Gy. Results. Comparative treatment planning shows that the treatment plan with rectal retraction significantly reduces (p<0.01) the volume of the rectal wall receiving high doses (equal to 70 Gy in 2 Gy fractions) in all patients. Conclusions. The proton boost treatment with retraction of rectum during treatment decreases the rectal dose substantially. This is expected to reduce rectal side effects.
External beam radiotherapy (EBRT) is widely accepted as a curative treatment modality for localised prostate cancer [Citation1]. In order to cure localised cancer of the prostate with radiotherapy, a high dose is needed [Citation2–4]. Different escalation schedules are used worldwide. By applying radiopaque markers in the prostate a reduced margin for the planning target volume (PTV) can be used which allows higher tolerable doses to be given. Still, the dose escalation is limited by the risk of complications, mainly to the rectum [Citation5]. Other approaches are the use of brachytherapy techniques given alone, or as a boost in combination with EBRT [Citation6].
Intensity Modulated Radiotherapy (IMRT) has improved the conformality of treatment delivery and reduced rectal toxicity [Citation7]. Proton treatment of the prostate is also utilised, either as a single treatment modality or in combination with conventional radiotherapy [Citation3,Citation8]. The advantageous dose distribution of proton beam radiotherapy for prostate cancer may result in reduced side effects compared to IMRT [Citation9]. With a single perineal proton beam it is possible to reduce the volume of the rectal wall included in the high dose region compared to conventional x-ray radiotherapy [Citation10]. All these high-precision radiotherapy techniques enable delivery of highly conformal dose distributions relative to the target volume and surrounding critical tissues. These conformal treatments with sharp dose gradients and escalated target dose are sensitive to setup errors and organ motion [Citation11]. In particular the proximity between the prostate and the rectal wall is problematic in this sense.
Holupka and coworkers [Citation12] have shown that, when inserting an ultrasound probe in the rectum for real-time imaging during EBRT, the probe also acts to fixate the prostate and reduce target motion. The probe also physically moves the rectal wall out of the high dose region. In high dose rate (HDR) brachytherapy, Åström and coworkers [Citation13] have used an ultrasound probe with a water stand-off, which was deflated after needle insertion to retract the anterior rectal wall from the prostate.
The standard radiotherapy treatment of localised prostate cancer in our department is a combination of EBRT, with a boost given either with HDR brachytherapy or protons. The proton treatment is given with the fixed horizontal proton beam at the The Svedberg Laboratory in Uppsala [Citation14]. At the proton treatment the prostate is positioned with high precision, using x-ray imaging of radiopaque gold markers previously implanted in the prostate [Citation15,Citation16]. The present study describes a method where the distance between the prostate and rectum is increased by retraction of the rectum in posterior direction. The purpose was to reduce the dose to the rectum without compromising target coverage and also to reduce movements of the prostate. A comparative dose planning study has been performed, with and without rectal retraction in order to quantify the dose reduction in the rectal wall.
Methods
Patients
Nine patients with biopsy proven and localised adenocarcinoma of the prostate were included in the test of the rectal retractor. Pre-treatment PSA was 4.3–12, Gleason Score 5–7 and T1–T3, all N0/NX/M0. The patients were treated with a proton boost to the prostate alone of 20 Gy given in 5 Gy fractions during four consecutive days, followed by a treatment with x-rays to a dose of 50 Gy in 2 Gy fractions five days per week.
Patient fixation and positioning
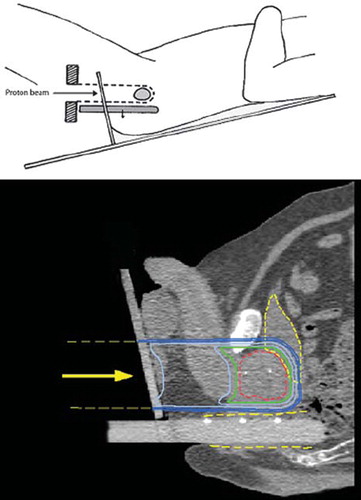
The patients were immobilised with a specially constructed fixation couch, where they were lying in lithotomy position in individually shaped vacuum pillows with the legs placed in adjustable leg supports. The surface of the perineum, where the proton beam enters, is irregular and variable, which makes it difficult to control the range of the protons such that the distal dose fall-off is adapted to the distal (cranial) end of the prostate. Therefore, the fixation couch was provided with a vertical Perspex plate onto which the patient's perineum is placed in firm contact. The proton beam thus passes through the Perspex plate on its way towards the prostate as illustrated in . Thereby the distance from the beam entrance to the distal end of the prostate can be determined in a reproducible way. The couch allows the patients to be tilted in the cranial-caudal direction to minimise irradiation of the rectal wall (). In order to displace the rectal wall posteriorly, a rectal retractor consisting of a cylindrical Perspex rod (diameter 1.5 cm) was inserted into the rectum. The rod was attached to the perineal Perspex plate and positioned horizontally to be parallel with the fixed horizontal proton beam. The rectum was retracted posteriorly (downwards) in order to maximise the separation between the prostate gland and the rectal wall. The rectal retractor has three radiopaque markers so that its position can be verified by CT-imaging for treatment planning and by x-ray imaging for treatment positioning. The patients were immobilised in the couch with identical rectal retraction both at the CT imaging before treatment and at each proton treatment. The patients were prescribed a laxative every day before fixation and gas was removed by suction from the rectum before imaging and treatment.
Figure 1. The patients were positioned in lithotomy position (upper panel) and the couch allowed the patients to be tilted in the cranially-caudally direction to closely match the horizontal proton beam with the rectal wall (i.e. the rectal wall and the proton beam should be parallel). In order to displace the rectal wall posteriorly, a cylindrical rod of Perspex was inserted into the rectum. The rectum was retracted posteriorly in order to maximize the separation between the prostate gland and the rectal wall. The lower panel shows a sagittal section where the prostate (red), bladder and rectal wall (yellow) are indicated together with the dose distribution. The isodose levels displayed are 95%, 90%, 70%, 50% and 30%. The radiopaque gold markers can be seen both in the prostate and in the retractor.
The magnitude of the retraction of the rectum relative to the prostate was determined in the CT-planning study by measuring the position of the markers in the prostate and markers in the rectal retractor. At each proton treatment, the patient's prostate was positioned using anterior-posterior and lateral x-ray images visualising the gold markers in the prostate and the markers in the rectal retractor. Thus it was possible to determine and reproduce the rectal retraction at the following treatments. The positioning of the target in the proton beam can be done with an accuracy of better than ± 1 mm by using the method described by Grusell and co-workers [Citation15]. This method was developed for positioning of intracranial targets by x-ray imaging of markers placed in the patient's skull and is in principle similar to the prostate positioning method used at x-ray treatments, where MV portal images are used to visualise the gold markers [Citation16].
For the x-ray treatment, the patients were positioned in a conventional supine position. MV portal images were used to verify the position by matching to bony structures. The gold markers could not be used to verify the position as the small marker size used for proton therapy made them hard to visualise on MV portal imaging. This means that for x-ray treatments, larger margins to the PTV were used.
Target volumes and risk organs
Before radiotherapy all patients underwent diagnostic MR imaging for guidance in delineation of the prostate gland in the CT-studies used for treatment planning. The targets delineated for proton treatment and x-ray treatment were not identical. The clinical target volume (CTV) for the proton boost was the prostate gland alone. To define the PTV a margin of 5 mm was applied around the CTV in all directions except near the rectum, where a margin of 2 mm was used. The organs at risk, the rectum wall and the bladder, were outlined. The rectum wall was defined as the space between the volumes limited by the outer rectal wall and the mucosa contour respectively.
The CTV for the x-ray treatment was either the prostate gland alone (two patients), or the prostate gland plus the seminal vesicles (seven patients). The seminal vesicles were included if any of the pre-treatment conditions PSA>10 ng/ml, Gleason>6 or >T2b were present. The PTV was defined as the CTV with a margin of 15 mm in lateral and ventral direction and 10 mm in cranial, caudal and dorsal direction. The larger PTV margin for x-ray treatment was motivated by the less accurate positioning of the prostate compared to proton therapy.
Treatment planning
Each patient was CT scanned with and without the rectal retractor before treatment at the same day. All nine patients were treated with the rectal retractor in the above described treatment position over the pelvic region with a slice thickness of 2 mm. The CT images were obtained from a Siemens Sensation 16 scanner. The image data were introduced into Helax-TMS [Citation17] (Treatment Management System, MDS Nordion Therapy System, Uppsala, Sweden). The system is provided with absorbed dose calculation algorithms for the different radiation modalities employed and has been subjected to adequate quality assurance tests [Citation18–20]. Dose distributions were presented as isodose contours in the CT slices and as dose volume histograms (DVH), which represent the distribution of dose in selected volumes of interest. The dose distributions for x-rays and protons were calculated using particle specific pencil beam algorithms [Citation19–21]. The x-ray treatment was given with a three field treatment technique using 15 MV x-rays from an Elekta Precise linear accelerator (Elekta AB, UK) equipped with a multileaf collimator (MLC). The proton beam treatment was realised with a single perineal proton beam with an individually shaped aperture. The range compensation filters were calculated to obtain a distal dose distribution conformal to the PTV with a 10 mm beam margin, allowing for range uncertainties in the variable entrance region (±5 mm), in bolus construction (±2 mm) and accelerator energy (±1 mm).
Comparative treatment planning study
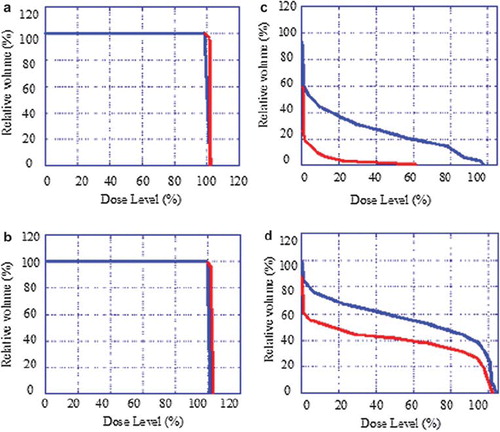
In order to investigate the effect of the rectal retractor on the dose distributions in the target and the rectum wall, two different proton plans were made for every patient, one with the rectal retractor (Proton_with) and one without (Proton_without). The D1% value and the volume of the rectal wall receiving at least 70 Gy for the combined proton and photon treatment (V70) were recorded. The V70 and the D1% values for the combined proton and photon treatment was calculated using the linear-quadratic effective dose model [Citation22]. For comparison the doses were recalculated and expressed in the corresponding total dose given in 2 Gy daily fractions (). A relative biological effectiveness (RBE) of 1.1 for the proton beam and an α/β-ratio of 3 were assumed in the calculation. The rectal volume receiving more than 70 Gy correlates significantly with rectal bleeding [Citation23].
Table I. The physical and the corresponding biological equivalent doses (BED) expressed in total dose given in 2 Gy daily fractions using an α/β-ratio of 3 and a RBE for protons of 1.1 for the two treatment plans.
The maximum dose and V70 were recorded when the dose distribution of both plans were covering the PTV with at least 95% of the prescribed dose. D1% is equal to the isodose level encompassing the 1 % of the rectal wall volume receiving the highest dose.
Results
The reproducibility in rectal retraction during treatment was determined for 27 recorded treatments, by comparing the retraction during treatment with the retraction during the CT-planning study acquisition. The retraction was determined as the difference (treatment vs CT-planning study acquisition) in distance between the markers in the rectal retractor and the markers in the prostate gland. The mean value was 0.4 mm with a standard deviation of 1.4 mm.
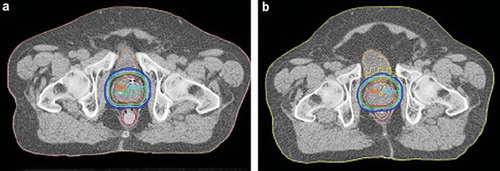
shows dose distributions for the two treatment plans with and without rectal retractor in a transverse section through the central part of the prostate for one representative patient. For the nine patients included in the treatment planning study the retraction of the rectal wall facing the prostate was on average 0.5 cm, with a range from 0.3 to 1.0 cm.
Figure 2. Dose distributions in a transverse section through the central part of the prostate for one representative patient for the two different treatment plans (a) with and (b) without the rectal retractor. The isodoses displayed are 95% (green), 90 and 70% (light blue), 50 and 30% (dark blue) of the prescribed dose.
DVHs for the PTV, CTV, the rectum wall and the bladder for one representative patient are presented in . The V70 values for the rectum wall for the nine patients are given in for the two different proton treatment plans in combination with the photon plan. The maximum dose to the rectum wall is also presented in . The treatment plan with the rectal retractor significantly reduced (p<0.05) the V70 value for the rectum wall. On average the volume reduction was 67% (range 5 to 96%). The maximum dose to the rectum wall was significantly lower (p<0.01) for the treatment plan with rectal retractor. On average, the dose reduction was 5% (range 1 to 10%).
Table II. The V70 in cm3 and the D1% for the rectum wall volume for nine patients, for two proton treatment plans in combination with the photon plan with and without the use of the rectal retractor, respectively.
Discussion
In this study, we have presented a method that significantly reduces the volume of the rectum receiving high doses when treating prostate cancer patients with a combination of protons and x-rays.
The therapeutic advantage of hypofractionation for the treatment of localised prostate cancer has been extensively discussed [Citation23] and recently, there has been a significant interest in pursuing hypofractionated schedules with external radiation therapy [Citation24–29]. However, the obvious concern with high-dose hypofractionated schedules is not so much prostate cancer cell kill as the potentially higher rates of late radiation toxicities [Citation23]. Kupelian and coworkers [Citation30] recommend 15 cm3 as the maximum rectal volume allowed to exceed the prescription dose, 70 Gy in 2 Gy fractions. This limit gives a mere 5% associated risk of rectal bleeding at two years follow-up [Citation30]. However, Kupelian and coworkers defined the rectum and not the rectum wall. Instead Vargas and coworkers [Citation31] has shown that V70 values between 5 cm3 and 15 cm3 for the rectal wall gives a 13% associated risk of chronic rectal complications of grade 2 or higher.
The retractor immobilises the rectal wall and prevents changes in gas and faeces filling. This reduces intrafractional motion of the rectum and ensures that the rectal wall does not move into the high dose region of the beam. The results of the 27 recorded treatments show that interfractional motion will not influence the results as the retraction of the rectum is well reproduced between fractions.
The total dose needed to cure prostate cancer is debated, and so is the value of α/β for prostate cancer. The biological equivalent dose (BED) with α/β 3 for the brachytherapy combination given at our department is 102 Gy in 2 Gy fractions and for the proton combination 87 Gy in 2 Gy fractions. BED with α/β is 116 Gy in 2 Gy fractions for the brachytherapy combination and 94 Gy in 2 Gy fractions for the proton combination. In Boston and Loma Linda [Citation3], a combination of x-rays (50.4 Gy) and protons (25.2 CGE) was given, to a total dose of approximately 75.6 CGE. Hara et al. [Citation8] treated the prostate with protons alone to 74 GyE in 37 fractions. Ishikawa and co-workers [Citation32] have treated the prostate with carbon ion radiotherapy to 66 GyE in 20 fractions. This fractionation corresponds to a BED of 83 Gy in 2 Gy fractions. The late toxicity (grade 2) for this treatment was 2% for the rectum and 5% for genitourinary system.
The method described in this work has so far only been used in proton beam treatments. However, this method can also be used for x-ray treatments, and with higher dose per fraction and shorter treatment time, the late rectal complication rates may be conserved or even lowered. In addition to possible radio-biologic gains, there are other obvious benefits to a hypofractionated treatment regime. The shorter time scale for treatment delivery and reduced numbers of delivered fractions lead to markedly improved patient convenience and substantial savings in resources.
Conclusion
In this study, we have presented a method that reduces the volume of the rectum receiving high doses when treating prostate cancer patients with proton beam radiotherapy. The method allows the patients to be treated with higher doses per fraction and thereby a shortened treatment time with lower or conserved late rectal complication rates.
Declaration of interest: The authors report no conflict of interest. The author alone are responsible for the content and writing of the paper.
References
- Pisansky TM. External-beam radiotherapy for localized prostate cancer. N Engl J Med 2006;355:1583–91.
- Pollack A, Zagars GK, Starkschall G, Antolak JA, Lee JJ, Huang E, . Prostate cancer radiation dose response: Results of the M. D. Anderson phase III randomized trial. Int J Radiat Oncol Biol Phys 2002;53:1097–105.
- Shipley WU, Verhey LJ, Munzenrider JE, Suit HD, Urie MM, McManus PL, . Advanced prostate cancer: The results of a randomized comparative trial of high dose irradiation boosting with conformal protons compared with conventional dose irradiation using photons alone. Int J Radiat Oncol Biol Phys 1995;32:3–12.
- Zietman AL, DeSilvio ML, Slater JD, Rossi CJ Jr, Miller DW, Adams JA, . Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: A randomized controlled trial. JAMA 2005;294:1233–9.
- Vargas C, Yan D, Kestin LL, Krauss D, Lockman DM, Brabbins DS, . Phase II dose escalation study of image-guided adaptive radiotherapy for prostate cancer: Use of dose-volume constraints to achieve rectal isotoxicity. Int J Radiat Oncol Biol Phys 2005;63:141–9.
- Vicini F, Vargas C, Edmundson G, Kestin L, Martinez A. The role of high-dose rate brachytherapy in locally advanced prostate cancer. Semin Radiat Oncol 2003;13:98–108.
- Mangar SA, Huddart RA, Parker CC, Dearnaley DP, Khoo VS, Horwich A. Technological advances in radiotherapy for the treatment of localised prostate cancer. Eur J Cancer 2005;41:908–21.
- Hara I, Murakami M, Kagawa K, Sugimura K, Kamidono S, Hishikawa Y, . Experience with conformal proton therapy for early prostate cancer. Am J Clin Oncol 2004;27:323–7.
- Mock U, Bogner J, Georg D, Auberger T, Potter R. Comparative treatment planning on localized prostate carcinoma conformal photon-versus proton-based radiotherapy. Strahlenther Onkol 2005;181:448–55.
- Benk VA, Adams JA, Shipley WU, Urie MM, McManus PL, Efird JT, . Late rectal bleeding following combined X-ray and proton high dose irradiation for patients with stages T3-T4 prostate carcinoma. Int J Radiat Oncol Biol Phys 1993;26:551–7.
- Mechalakos JG, Mageras GS, Zelefsky MJ, Lyass O, van Herk M, Kooy HM, . Time trends in organ position and volume in patients receiving prostate three-dimensional conformal radiotherapy. Radiother Oncol 2002;62:261–5.
- Holupka EJ, Kaplan ID, Burdette EC, Svensson GK. Ultrasound image fusion for external beam radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys 1996;35:975–84.
- Astrom L, Pedersen D, Mercke C, Holmang S, Johansson KA. Long-term outcome of high dose rate brachytherapy in radiotherapy of localised prostate cancer. Radiother Oncol 2005;74:157–61.
- Montelius A, Blomquist E, Naeser P, Brahme A, Carlsson J, Carlsson AC, . The narrow proton beam therapy unit at the Svedberg Laboratory in Uppsala. Acta Oncol 1991;30:739–45.
- Grusell E, Montelius A, Russell KR, Blomquist E, Pellettieri L, Lilja A, . Patient positioning for fractionated precision radiation treatment of targets in the head using fiducial markers. Radiother Oncol 1994;33:68–72.
- Dehnad H, Nederveen AJ, Van der Heide UA, Van Moorselaar RJA, Hofman P, Lagendijk JJW. Clinical feasibility study for the use of implanted gold seeds in the prostate as reliable positioning markers during megavoltage irradiation, Radiother Oncol 2003;67:295–302
- Jung B, Montelius A, Dahlin H, Ekström P, Ahnesjö A, Högström B, . The conceptual design of a radiation oncology planning system. Comput Methods Programs Biomed 1997;52:79–92.
- Montelius A, Jung B, Rikner G, Murman A, Russell K. Quality assurance tests of the TMS-radix treatment planning system. Breit A. Advanced radiation therapy tumour response monitoring and treatment planning. Berlin, Heidelberg: Springer-Verlag; 1992. 523–7.
- Russell KR, Isacsson U, Saxner M, Ahnesjö A, Montelius A, Grusell E, . Implementation of pencil kernel and depth penetration algorithms for treatment planning of proton beams. Phys Med Biol 2000;45:9–27.
- Ahnesjo A, Saxner M, Trepp A. A pencil beam model for photon dose calculation. Med Phys 1992;19:263–73.
- Dose formalism and models in helax-TMS. Helax AB. Uppsala, Sweden: 1998.
- Fowler JF. Fractionated radiation therapy after Strandqvist. Acta Radiol Oncol 1984;23:209–16.
- Kupelian PA, Reddy CA, Klein EA, Willoughby TR. Short-course intensity-modulated radiotherapy (70 Gy at 2.5 Gy per fraction) for localized prostate cancer: Preliminary results on late toxicity and quality of life. Int J Radiat Oncol Biol Phys 2001;51:988–93.
- Brenner DJ. Hypofractionation for prostate cancer radiofherapy-what are the issues? Int J Radiat Oncol Biol Phys 2003;57:912–4.
- Brenner DJ, Hall EJ. Fractionation and protraction for radiotherapy of prostate carcinoma. Int J Radiat Oncol Biol Phys 1999;43:1095–101.
- Brenner DJ, Martinez AA, Edmundson GK, Mitchell C, Thames HD, Armour EP. Direct evidence that prostate tumors show high sensitivity to fractionation (low alpha/beta ratio), similar to late-responding normal tissue. Int J Radiat Oncol Biol Phys 2002;52:6–13.
- Fowler J, Chappell R, Ritter M. Is alpha/beta for prostate tumors really low? Int J Radiat Oncol Biol Phys 2001;50:1021–31.
- Fowler JF, Ritter MA, Chappell RJ, Brenner DJ. What hypofractionated protocols should be tested for prostate cancer? Int J Radiat Oncol Biol Phys 2003;56:1093–104.
- Logue JP, Cowan RA, Hendry JH. Hypofractionation for prostate cancer. Int J Radiat Oncol Biol Phys 2001;49:1522–3.
- Kupelian PA, Reddy CA, Carlson TP, Willoughby TR. Dose/volume relationship of late rectal bleeding after external beam radiotherapy for localized prostate cancer: Absolute or relative rectal volume? Cancer J 2002;8:62–6.
- Vargas C, Martinez A, Kestin LL, Yan D, Grills I, Brabbins DS, . Dose-volume analysis of predictors for chronic rectal toxicity after treatment of prostate cancer with adaptive image-guided radiotherapy. Int J Radiat Oncol Biol Phys 2005;62:1297–308
- Ishikawa H, Tsuji H, Kamada T, Yanagi T, Mizoe JE, Kanai T, . Carbon ion radiation therapy for prostate cancer: Results of a prospective phase II study. Radiother Oncol 2006;81:57–64.