Abstract
Differences in Nordic cancer patient survival observed today originate from the 1970s, but were first identified in a mortality prediction from 1995. This paper provides timely comparisons of survival using NORDCAN, a database with comparable information from the Nordic cancer registries. Elucidation of the differences is important when monitoring cancer care generally and evaluating the impact of cancer plans. Material and methods. The NORDCAN database 1964–2003 with follow-up for death through 2006, was used to analyse incidence, mortality, and survival for all NORDCAN cancer sites. We analysed 5-year relative survival and excess mortality rates in the first three months and 2–5 years after diagnosis. Results. The time trends in survival 1989–2003 were largely similar between the Nordic countries with increases in 14 sites among men and 16 among women. In all countries the excess mortality rates were highest in the first three months after diagnosis, but decreased to similar levels across all countries 2–5 years after diagnosis. Comparing countries excess mortality was highest in Denmark irrespective of follow-up period. Lower survival was observed for Danish cancer patients in 23 of the 33 cancer sites in men and 26 of 35 sites in women. Low and similar levels of survival were observed for cancers of the oesophagus, lung, liver and pancreas, while an 8–10 percentage point difference in survival was found between countries for colorectal cancer. Conclusion. The notable differences in Nordic cancer patient survival can be linked to national variations in risk factors, co-morbidity, and the implementation of screening. Improved treatment and primary prevention, in particular the targeting of tobacco and alcohol use, is required to improve cancer control. The recently-initiated cancer plans in Denmark and Norway are yet to show an observable effect on the corresponding cancer survival.
Survival differences among Nordic cancer patients have been reported in several publications [Citation1–6]. The first comprehensive account of differences across all primary sites, published in 1964, reported a lower survival in Finland compared to the other Nordic countries [Citation1]. In contrast, the most recent Nordic analysis from 1995 [Citation2] showed Danish patients to have the lowest survival. Part of the data were re-analysed in a European setting by the EUROCARE group, which confirmed the findings and demonstrated that the Nordic countries, except Denmark, had the highest cancer survival in Europe [Citation7,Citation8].
The repeated observation of a lower survival in Denmark pointed to the need for a comprehensive cancer control plan in Denmark, of which the first was launched in the year 2000, and a follow-up plan in 2004 [Citation9,Citation10]. The Cancer Society and other professional societies in oncology and diagnostics initiated working groups some years prior to the launch of the plan, leading to a partial implementation of the recommendations ahead of the official launch. When launched, the plan lead to a fast renewal and expansion of radiotherapy equipment, a boost in modern imaging techniques, implementation of new chemotherapeutic regimes, and a substantial financial commitment from the government for cancer care. The second plan included organisation of surgery and screening and packages securing a fast track of patients and minimal waiting times.
The Finnish government appointed in April 1952 a committee commissioned “to make a proposal for intensification of the control of malignant diseases”. In the summary of the committee's report the following was stated: “There exists as yet no reliable preventive method for the control of malignant diseases”. Therefore, the committee recommended intensification of early diagnosis, an increase in therapeutic facilities and a promotion of research [Citation11]. A cancer control plan for preventing cancer was then subsequently formulated in Finland already in 1984, highlighting the need for health education, research, tobacco and alcohol control, better nutrition, and protection against environmental cancer risks [Citation11], and a comprehensive plan adding diagnostics and therapy to the previous was launched in March 2010. In Norway, a government-backed comprehensive cancer plan with 20 specific tasks in the domains of prevention, early diagnosis and screening, through to improved care and better organisation of care, education, financial matters, and research was published in 1997 [Citation12]. While in Sweden, the regional and national cancer care programmes based on clinical guidelines have since the 1970s been lead, updated, and followed by the various health regions. This is a first very important step in a cancer plan, and should become part of a comprehensive approach, from prevention and early diagnosis through to rehabilitation and palliation. Further, sufficient funding and government support are required to ensure the goals of a WHO cancer plan strategy are reached. Cancer was given limited focus in Iceland in the second health plan implemented in 2001.
To assess the impact of the plans it is necessary to follow both the care and quality of life of the patients and the outcomes of a comprehensive approach to cancer, for which incidence, mortality, and survival are essential measures. However, it is insufficient to judge these in national settings, and international benchmarking is needed [Citation13], and may be assessed within a reasonably short period of follow-up. Hence a comparable dataset is paramount, and for this purpose the NORDCAN database [Citation14,Citation15] was established to serve as a basis for research and a tool for monitoring cancer in the Nordic countries. The aim of this study is to give an overview of Nordic cancer patient survival across cancer sites and to study the current trends in survival some 10 years after the last comprehensive review [Citation2]. The present paper summarises the most recent survival results from a series of site-specific papers [Citation16–27] with a view to assessing the impact on survival of a shifting focus on cancer control in the Nordic countries during the last decade.
Material and methods
We compiled the results for the time period 1999–2003 followed through 2006 as analysed in the series of papers studying survival trends for cancer patients in the Nordic countries 1964–2003 [Citation16–27]. A uniform set of methods was used in these reports, as explained in detail in Engholm et al. [Citation16]. In brief, we used the NORDCAN database, and hence the data have been checked and converted to well-defined entities. We included all cancer patients diagnosed with malignant neoplasms (ICD 10: C00-C95+D09.0+D41.4+D32-33+D42-43) excluding non-melanoma skin cancer (ICD10: C44+C46.0) 1999–2003 in Denmark, Finland, Iceland, Norway, and Sweden, and supplemented the cancer records with individual follow-up for death and emigration up to the end of 2006. All sites combined (excluding non-melanoma skin cancer) were analysed in three ways, with and without adjustment for case-mix, and with another case-mix adjustment which excluded breast and prostate cancer for women and men, respectively [Citation16,Citation27]. We used a hybrid analysis combining period and cohort survival [Citation28] for the period 1999–2003. Country-specific life tables were used to calculate the expected survival. Age-standardisation was performed using the standard weight distributions for cancers (ICSS standards) as in the EUROCARE-4 analysis [Citation29]. Patients were followed until death, emigration or loss to follow-up, or to the end of 2006. Excess mortality rates were stratified into short intervals after diagnosis: the first month, the first three months, 1–3 months, 4–12 months and yearly intervals thereafter.
In order to evaluate if 5-year relative survival ratios could be predicted by excess mortality rates in the first three months following diagnosis across sites, we plotted the age-standardised 5-year relative survival ratio against the excess mortality rate in the first three months for the 15 largest cancer sites 1999–2003 for each sex after breast and prostate cancer were excluded. This was applied for each combination of country and sex. For each plot a LOESS curve [Citation30] was fitted and the sex-specific LOESS-curves for the five countries were compared. The comparisons were made in figures with the excess mortality rates on a logarithmic axis.
The trends in survival were assessed by comparing the 5-year relative survival ratios in 1999–2003 with the two preceding periods 1989–93 and 1994–98. We present 5-year age-standardised relative survival ratios (RS) for 1999–2003 and a graphical symbol (arrows) of time trends. Further tables are presented describing the highest and lowest 5-year age-standardised relative survival ratios by country, site and sex, and tables on excess mortality rates for the follow-up periods, the first three months and 2–5 years following diagnosis.
Results
and for men and women respectively, present an overview of the 5-year age-standardised relative survival ratios in the Nordic countries by site for the most recent period 1999–2003, and the trends in survival observed during the three calendar periods 1989–1993, 1994–1998, 1999–2003, with the direction of the trend indicated by arrows. Due to the small numbers of cancer cases in Iceland it was often difficult to assess the temporal development.
Table I. Five-year age-standardised relative survival (%) (RS) with 95% confidence intervals (CI) by site and country for patients diagnosed 1999–2003 and trends in survival 1989–2003. Nordic cancer survival study: Men.
Table II. Five-year age-standardised relative survival (%) (RS) with 95% confidence intervals (CI) by site and country for patients diagnosed 1999–2003 and trends in survival 1989–2003. Nordic cancer survival study: Women.
For cancers of the oesophagus, colon, rectum, prostate, kidney, and Hodgkin lymphoma among men, survival has increased in all countries since 1989, as also seen for other haematological malignancies, cancers of the brain, tongue, pharynx, and melanoma of the skin. Cancer survival in women has increased in all countries since 1989 for Hodgkin lymphoma, and cancers of the breast, corpus uteri and brain, and increased in most countries for cancers of the stomach, colon, rectum, lung, ovary, kidney, bladder, as well as other haematological malignancies and melanoma of the skin.
In general, the 5-year age-standardised survival ratios for cancers in lip, oral cavity and pharynx in men and women were lowest in Denmark with the exception of pharyngeal cancer in Norway. The Danish deficit relative to the other countries was statistically significant for cancers of the tongue and mouth, with the exception of Finland (males). The same pattern, with lower survival in Denmark, was seen for cancers of the digestive tract with the exception of cancers of the gallbladder and extrahepatic biliary ducts and pancreas in both sexes, with equally poor survival in all countries, and for cancer of the liver in women. For cancers of the respiratory tract, survival was fairly similar across countries although the poor survival associated with lung cancer was higher in Norway, Iceland and Sweden for both sexes, and in Finnish women. Ovarian cancer among the sex-specific cancers and female breast cancer reflect a general pattern of lower survival in Denmark. In men, survival after testicular cancer was high in all countries (RS: 88–94%), whereas Denmark had a lower survival ratio for prostate cancer (RS: 53%) compared to the other countries (RS: 78–86%). Kidney cancer survival was considerably lower in Denmark (40–41%) compared to the other countries (51–62%). Bladder cancers were observed to have the same pattern with lower survival among Danish men (RS: 71%) and women (RS: 62%) whereas the survival in the other countries with few exceptions was rather homogenous (RS-men: 73–76%; RS-women 68–72%). Survival after diagnoses of melanoma of the skin was a little lower in Norway, but the survival was high generally, around 90% in women, and a little lower in men. A similar pattern between countries was seen for cancers of the eye, thyroid, and bone, with a somewhat lower survival in Denmark, most evident among women, with smaller differences in survival following diagnoses of cancers of the brain and soft tissues. The haematological malignancies however demonstrated a much more uniform 5-year survival for non-Hodgkin lymphoma in both sexes and in men for Hodgkin lymphoma and multiple myeloma.
Of the 33 cancer sites examined in men, Denmark had the lowest 5-year relative survival for 23 sites, Finland in six, and Norway in seven. Sweden did not rank lowest for any cancer () and had the highest survival for 16 cancer sites followed by Finland (Citation15), Norway (Citation7) and Denmark (Citation1). For 12 of the 33 sites, the percentage point differences in 5-year relative survival were 10 or greater, including cancers of the prostate, small intestine, penis and other genital organs, kidney, tongue, eye, thyroid, stomach, bone, colon as well as other leukaemia, and to a lesser extent for common cancers such as lung and bladder, as well as melanoma and most haematological malignancies. For women, the pattern was similar, with Denmark ranking lowest for 26 of 35 sites (). For 16 sites, the percentage point differences were 10 or higher, and as for men, included cancers of the tongue, kidney, stomach, small intestine, colon, thyroid, bone and other leukaemia, as well as cancers of the neoplasms of the lip, mouth, pharynx, rectum, larynx, bladder, and ovary. Comparing the highest 5-year age-standardised survival ratios in the Nordic countries between sexes, large differences – from 13 to 19 percentage points – were observed for cancers of the tongue, mouth, pharynx, and brain. Differences between sexes of 6 to 9 percentage points were seen for cancers of the lip, salivary glands, oesophagus, rectum, nose, pleura, melanoma, eye, thyroid, non-Hodgkin, and acute leukemia. For the remaining 16 sites, survival differences were 5 percentage points or less for frequent cancers such as lung, bladder, kidney, and colon. Iceland is not included in any of these comparisons because of the rather unstable estimates for most cancers. However, on considering the five most frequent cancer sites – for which the estimates are most stable – the Icelandic survival ratios tended to be on the higher side. Male survival ranked first for cancers of the lung (along with Swedish males), kidney and bladder, while no survival ranked lowest among the sites under consideration. Icelandic females had the highest survival for breast cancer among the Nordic countries, and the lowest survival following cancer of the corpus uteri.
Table III. Lowest and highest 5-year age-standardised relative survival ratio (RS) 1999–2003 in the Nordic countries* and percentage point difference. Nordic cancer survival study: Men.
Table IV. Lowest and highest 5-year age-standardised relative survival ratio (RS) 1999–2003 in the Nordic countries* and percentage point difference. Nordic cancer survival study: Women.
and present the excess mortality rates 1999–2003 in follow-up intervals spanning the first three months and 2–5 years after diagnosis. The rates during the first three months after diagnosis reflect the overall observed pattern for the relative survival, inasmuch that Danish men and women have higher excess mortality rates than their Nordic neighbours generally, and the male-female differences in survival are most evident in the excess mortality early in the follow-up (). The absolute differences appear to have largely disappeared 2–5 years after the diagnosis, but the relative differences remain or increase slightly for the more frequent cancers of the colon, rectum, lung, breast (females) and melanoma (). A few cancer sites, including cancers of the oesophagus, liver, gallbladder and biliary tract, pancreas, lung, pleura, and multiple myeloma, are notable for still – contrary to remaining sites – to have high absolute excess mortality rates after two years.
Table V. Excess mortality rates 1999–2003 per 100 person years; Follow-up interval first three months. Nordic cancer survival study.
Table VI. Excess mortality rates 1999–2003 per 100 person years; Follow-up 2–5 years. Nordic cancer survival study.
presents the 5-year relative survival ratios plotted against the excess mortality rates in the first three months for the 15 most frequent cancer sites excluding prostate and female breast cancer for each combination of country and sex, with the fitted curves compared between countries separately for each sex in , with excess mortality on a logarithmic scale. The shapes of the curves are rather similar between countries. Differences were smallest for cancer sites associated with low survival (and high excess mortality). Using weights from the case-mix adjustment in the curve-fitting did not alter the curves substantially (not shown).
Figure 1. Five-year relative survival plotted against excess mortality rates in the first three months following diagnoses in 1999–2003 for cancer patients by country and sex with LOESS curves for the 15 most common cancer sites excluding prostate and breast cancer. Nordic cancer survival study.
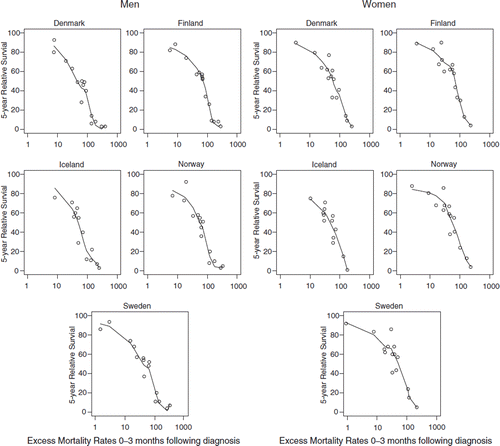
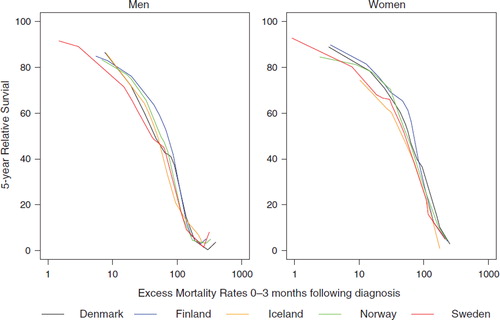
Figure 2. Country-specific fitted LOESS curves to plots of 5-year relative survival against excess mortality rates in the first three months following diagnosis in 1999–2003 for male and female cancer patients of the 15 most common cancer sites excluding prostate and breast. Nordic cancer survival study.
Discussion
We have compiled the survival and excess mortality data from a series of papers evaluating the cancer incidence, mortality, relative survival and excess mortality from 1964–2003 in the Nordic countries [Citation16–27]. We studied 1999–2003 to limit the analysis to timely and comparable incidence data on cancer available from each of the Nordic countries with a follow-up of the patients until the end of 2006. Our aim is to provide an overview and a summary of findings, inasmuch that such a Nordic comparison is most relevant for the study of the potential impact of established cancer control plans and the implementation of new treatment modalities at the end of the last century and the beginning of the current one. It must be stressed however, that comparisons of national relative survival ratios and excess mortality rates must also consider the cancer incidence and the case-mix [Citation27]. This is particularly important when studying all cancer sites combined, and for specific cancers where screening (breast, cervix) or other early diagnostic measures like PSA (prostate) or Haemoccult for blood in the stools (colorectal) are implemented on a population basis, be it part of an organised activity or otherwise. It must also be noted that from a clinical point of view, our data are comparable only at a rather crude level. Cancer registry data are usually unable to record new diagnostic innovations at the time they are implemented, and thus subgroups of cancers within an ICD category for which successful treatments exist, are often underemphasised. Perhaps more importantly in survival comparisons, staging is rarely recorded and reported in a uniform and comprehensive way. Hence we could not include stage and histology in the present analysis. However, the Nordic cancer registry data and its compilation into the NORDCAN database [Citation15] are renowned for their high quality and completeness, with excellent register-based follow-up of all cancer patients, and are thus ideally suited to the study the effect of cancer control planning. The registry data provides an unbiased population-based perspective of the survival of cancer patients, and one that is vital for the planning and monitoring of health care, serving the clinical specialties as they look beyond clinical trials to population interventions.
A large number of clinical publications have shown the relevance of short-term survival, especially following surgical interventions. The measure is also highly relevant in the study of the impact of national cancer plans [Citation13] and for cancers where surgical interventions are commonly not undertaken. Co-morbidity may be a factor capable of explaining some of the survival differences in Denmark for colorectal, prostate and ovarian cancer patients [Citation31–33] as well as diabetics with heart diseases [Citation34], and in terms of shared risk factors, the effect of tobacco and alcohol consumption, as discussed in the cancer-specific papers on survival [Citation17–27]. Quitting alcohol and tobacco use before elective surgery has been shown to lead to lower peri-operative morbidity and better recovery in a review paper [Citation35], while a Dutch study concluded that co-morbidity might lead to less invasive and less optimal cancer treatment being used [Citation36]. The level of smoking in Denmark has from the 1970s been higher than in the other Nordic countries, except among Norwegian women in 2000–2004 () and the Icelandic population in the 1960s [Citation37–39]. Before the 1980s, the average tobacco consumption per capita was highest in Denmark, with Finland and Norway close behind, but with very differing smoking patterns seen between countries. A large part of the Danish consumption patterns could at that time be attributed to pipe, cigars and cigarillos, while Finns mostly smoked strong Russian-type cigarettes and Norwegians, hand-rolled cigarettes. In Sweden, the consumption of smokeless tobacco has been historically high [Citation38,Citation40]. The Swedish snus has been described as an interesting but controversial experience amongst believers in harm reduction, and reducing exposure to tobacco [Citation41]. However this avenue to tobacco control has been carefully assessed and warned against by Swedish public health researchers [Citation42,Citation43]. Cigarette smoking in Finland was high already in the 1920s [Citation40], but from the mid-1960s anti-smoking legislation and campaigns started in Finland [Citation44]. In more recent years cigarette smoking has become the key mode of tobacco consumption in the Nordic countries, making the OECD registrations of daily tobacco smokers (in ) a more comparable measure of risk assessment.
Figure 3. Trends in the proportion of daily smokers in the Nordic countries by sex. (Sources: lines depict data from OECD (reference [Citation36]) while the circles represent data from reference [Citation38]).
![Figure 3. Trends in the proportion of daily smokers in the Nordic countries by sex. (Sources: lines depict data from OECD (reference [Citation36]) while the circles represent data from reference [Citation38]).](/cms/asset/1d0dc830-db9c-49f7-bb4b-b359e915877a/ionc_a_480636_f0003_b.jpg)
Trends in average alcohol consumption indicate that Danish consumption has been double that estimated in the other countries (except Finland) () [Citation37,Citation45]. Although not the only contributing factors, the patterns of tobacco and alcohol consumption are in accordance with trends in life expectancy [Citation16], as well as the observed patterns of cancer incidence and survival, and probably play an independent role in explaining some of the survival variations observed between the countries.
Figure 4. Trends in alcohol consumption in the Nordic countries (Source: reference [Citation36]).
![Figure 4. Trends in alcohol consumption in the Nordic countries (Source: reference [Citation36]).](/cms/asset/29327445-70ba-41dd-98cb-de2d9265913b/ionc_a_480636_f0004_b.jpg)
The excess mortality rate for the first months after diagnosis has been demonstrated to be a good indicator of the level and ranking of the 5-year age-standardised survival rates in our Nordic study [Citation13,Citation46]. The excess mortality was much higher in the 1960s through to the late-1980s in the first month after diagnosis, with the difference between the first and the following two months after a cancer diagnosis becoming rather minor. Hence the excess mortality for the first three months after a diagnosis in the 1990s and thereafter will be more informative and stable to study.
The LOESS curves fitted to plots of 5-year relative survival by excess mortality 0–3 months after diagnosis for the 15 most common cancer sites (excluding breast and prostate cancer) ( and ), show a remarkably similar shape between countries for both sexes. The variation in 5-year relative survival at an excess mortality ratio of around 10 is only around 5 percentage points between countries, whereas the differences for an excess death ratio of 50 are larger, particularly among men. The largest differences relate to colorectal and kidney cancers. The similar pattern and shape of the curves between countries and by sex, advocate for the use of excess mortality 0–3 month as an early indicator of the impact of new actions designed to improve cancer control. The curves for Sweden are consistently lower and shifted a little to the left relative to the other countries, an effect likely related to missing death certificate initiated cancers.
The correlation between the early mortality and the 5-year relative survival is an important observation for the monitoring of the impact of the implementation of cancer plans, with the reservation that the impact of prevention and health promotion is likely to be observable only after several decades, and only for those actions that have a large impact on the general population, such as smoking cessation. Although a general observation is that Danish cancer patients fare worse than their Nordic counterparts, it is noted that the time trends in survival by country – with a few exceptions such as prostate and breast cancer – are rather parallel after an initial period of approximately 6–12 months after diagnosis. It thus seems that, other than the initial survival deficit, the health care system in Denmark is able to deliver treatment and care, and to capitalise on new developments in diagnosis and treatment, as in the other countries.
Our study highlights both the similarities and the differences in survival among cancer patients in the Nordic countries. Where findings are consistent between the sexes and countries, they most likely reflect what is achievable at a population level today, considering the wealth of the Nordic countries and the organisation of health care. Apart from prostate and breast cancer [Citation20,Citation22], where the survival was high but heavily influenced by screening and diagnostic activities, it is noteworthy that patients diagnosed with melanoma of skin, Hodgkin lymphoma and cancers of the testis, thyroid and lip all have 5-year relative survival ratios over 80%. Only a few cancer patient groups – specifically those diagnosed with cancers of the oesophagus, gallbladder and biliary ducts, lung, pleura, liver, and pancreas – present with survival ratios below 20%, while diagnoses of acute leukaemia, multiple myeloma, stomach, and ovary are associated with levels of survival below 50%. Such observations are helpful when making prioritised decisions regarding the strategies for tackling the cancer problem. For cancers associated with very poor prognosis, it is necessary to focus more on prevention and research into modifiable risk factors that may prevent the disease. The case for further tobacco and alcohol control is obvious for those cancer types with the lowest survival. With the general survival pattern in mind, prevention, especially a healthier life style with less tobacco and alcohol, would seem a strategy necessary to enforce as part of cancer control planning [Citation47], in particular in Denmark, where survival is lowest and excess mortality highest. There is room for improvement of the survival for colon and rectal cancer in all countries, but most evidently in Denmark. Screening and early diagnosis are obvious possibilities for these cancers [Citation18,Citation48,Citation49], combined with improved surgery.
Survival is an important measure of early diagnosis, treatment and the quality of care of cancer patients; however it is not sufficient for the evaluation of cancer control. Survival only partially explains results pertaining to treatment after the initial diagnosis. From the perspective of the individual, it is much more important not to develop the disease, that is, either to be cured following treatment of a precancerous lesion or to avoid contracting cancer at all. Hence, if a cancer control plan is to be shown effective, we should expect a decline in incidence and mortality, and an increase in survival. However, with success in avoiding cancers at an early age, coupled with global demographic changes (increases in life expectancy, population ageing), both the future numbers of new cancer cases and deaths may increase if the current age-specific incidence and mortality remain unchanged [Citation50], even if we manage to better control known risk factors for cancer, as described in the site-specific papers [Citation17–27]. It is on the other hand quite plausible that the results of effective preventive interventions will lead to a better general health [Citation47], and thus less co-morbidity for those contracting cancer, and subsequently, higher success rates in terms of therapy and supportive care during the initial treatment and beyond.
We initiated this study to provide a comprehensive overview of the survival of Nordic cancer patients, with a view to be able to study the effects of cancer control. Despite cancer plans formulated and put in action in both Denmark and Norway, we cannot as yet see a direct impact on incidence, mortality, or survival. It may be too early to see the results compared to Sweden and Finland. The cancer care plans in Sweden that improved the quality of cancer care, and the Finnish prevention plan that led to an improved general health, were instigated several decades ago. In terms of observable changes in cancer incidence and mortality, prevention activities usually surface many years or several decades after initiation, whilst new effective therapies will show up rather rapidly, if applied to the majority of patients. We noted that the survival trends seem to be parallel for most sites with country differences driven by the excess mortality right after diagnosis. In the early years this was especially high during the first month of follow-up, but in the most recent periods the excess mortality in the first month is of a similar order of magnitude to that observed in the following two months, although still highest in Denmark for the majority of cancer sites. In summary, the differences in survival between the Nordic countries likely relate to a range of host and institutional determinants. They include a varying prevalence of exposure to key risk factors such as tobacco and alcohol, and resultant differences in co-morbidity, as well as differences in the stage at presentation at diagnosis, and in the treatment and management of the disease.
Acknowledgements
The Nordic Cancer Union (NCU) has financially supported the development of the NORDCAN database and program, as well as the survival analyses in this project.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Cutler SJ. International symposium on end results of cancer therapy. Natl Cancer Inst Monogr 1964;15:1–446.
- Engeland A, Haldorsen T, Tretli S, Hakulinen T, Hörte LG, Luostarinen T, . Prediction of cancer mortality in the Nordic countries up to the years 2000 and 2010, on the basis of relative survival analysis. A collaborative study of the five Nordic Cancer Registries. APMIS 1995;103(Suppl 49):1–161.
- Engeland A, Haldorsen T, Dickman PW, Hakulinen T, Möller TR, Storm HH, Tulinius H. Relative survival of cancer patients – a comparison between Denmark and the other Nordic countries. Acta Oncol 1998;37:49–59.
- Storm HH, Dickman PW, Engeland A, Haldorsen T, Hakulinen T. Do morphology and stage explain the inferior lung cancer survival in Denmark? Eur Respir J 1999;13:430–5.
- Christensen LH, Engholm G, Ceberg J, Hein S, Perfekt R, Tange UB, . Can the survival difference between breast cancer patients in Denmark and Sweden 1989 and 1994 be explained by patho-anatomical variables? – a population-based study. Eur J Cancer 2004;40:1233–43.
- Christensen LH, Engholm G, Cortes R, Ceberg J, Tange U, Andersson M, . Reduced mortality for women with mammography-detected breast cancer in east Denmark and south Sweden. Eur J Cancer 2006;42:2773–80.
- Coleman MP, Gatta G, Verdecchia A, Esteve J, Sant M, Storm H, . EUROCARE-3 summary: Cancer survival in Europe at the end of the 20th century. Ann Oncol 2003;14(Suppl 5):v128–49.
- Sant M, Allemani C, Santaquilani M, Knijn A, Marchesi F, Capocaccia R, . EUROCARE-4. Survival of cancer patients diagnosed in 1995–1999. Results and commentary. Eur J Cancer 2009;45:931–91.
- The National Cancer Plan – Denmark 2000. Summary and recommendations. Copenhagen, Denmark: The National Board of Health; 2000.
- National Cancer Plan II – Denmark 2005. National Board of Health recommendations for improving cancer health care services. Copenhagen, Denmark: The National Board of Health; 2005.
- Cancer, occurrence, risk factors and prevention. Report of the Finnish Cancer Committee 1984. Helsinki Finland; 1984.
- Care and knowledge, Norwegian Cancer Plan. A report from a committee for a national cancer plan established by the Ministry for Health and Social Affairs. Oslo: NOU; 1997. 20.
- Storm HH, Gislum M, Engholm G. [Cancer survival before and after initiating the Danish Cancer Control plan] (in Danish). Ugeskr Laeger 2008;170:3065–9.
- Engholm G, Ferlay J, Christensen N, Bray F, Gjerstorff ML, Klint Å, . NORDCAN: Cancer incidence, mortality and prevalence in the Nordic countries, Version 3.4. Association of Nordic Cancer Registries. Danish Cancer Society; 2009. Available from: http://www.ancr.nu.
- Engholm G, Ferlay J, Christensen N, Bray F, Ólafsdóttir E, Klint Å, . NORDCAN: A Nordic tool for cancer information, planning, quality control, and research. Acta Oncol 2010;49:725–36.
- Engholm G, Gislum M, Bray F, Hakulinen T. Trends in the survival of patients diagnosed with cancer in the Nordic countries 1964–2003 followed up to the end of 2006. Material and methods. Acta Oncol 2010;49:545–60.
- Hakulinen T, Tryggvadóttir L, Gislum M, Storm HH, Bray F, Klint Å, . Trends in the survival of patients diagnosed with cancers of the lip, oral cavity, and pharynx in the Nordic countries 1964–2003 followed up to the end of 2006. Acta Oncol 2010;49:561–77.
- , Klint ÅEngholm G, Storm HH, Tryggvadóttir L, Gislum M, Hakulinen T, . Trends in the survival of patients diagnosed with cancer of the digestive organs in the Nordic countries 1964–2003 followed up to the end of 2006. Acta Oncol 2010;49:578–607.
- Hakulinen T, Engholm G, Gislum M, Storm HH, Klint Å, Tryggvadóttir, . Trends in the survival of patients diagnosed with cancers in the respiratory system in the Nordic countries 1964–2003 followed up to the end of 2006. Acta Oncol 2010;49:608–23.
- Tryggvadóttir L, Gislum M, Bray F, Klint Å, Hakulinen T, Storm HH, . Trends in the survival of patients diagnosed with breast cancer in the Nordic countries 1964–2003 followed up to the end of 2006. Acta Oncol 2010;49:624–31.
- Klint Å, Tryggvadóttir L, Bray F, Gislum M, Hakulinen T, Storm HH, . Trends in the survival of patients diagnosed with cancer in the female genital organs in the Nordic countries 1964–2003 followed up to the end of 2006. Acta Oncol 2010;49:632–43.
- Bray F, Klint Å, Gislum M, Hakulinen T, Engholm G, Tryggvadóttir L, . Trends in the survival of patients diagnosed with male genital cancers in the Nordic countries 1964–2003 followed to the end of 2006. Acta Oncol 2010;49:644–54.
- Engholm G, Hakulinen T, Gislum M, Tryggvadóttir L, Klint Å, Bray F, . Trends in the survival of patients diagnosed with kidney or urinary bladder cancer in the Nordic countries 1964–2003 followed up to the end of 2006. Acta Oncol 2010;49:655–64.
- Tryggvadóttir L, Gislum M, Hakulinen T, Klint Å, Engholm G, Storm HH, . Trends in the survival of patients diagnosed with malignant melanoma of the skin in the Nordic countries 1964–2003 followed up to the end of 2006. Acta Oncol 2010;49:665–72.
- Bray F, Engholm G, Hakulinen T, Gislum M, Tryggvadóttir L, Storm HH, . Trends in survival of patients diagnosed with cancers of the brain and nervous system, thyroid, eye, bone, and soft tissues in the Nordic countries 1964–2003 followed up to the end of 2006. Acta Oncol 2010;49:673–93
- Storm HH, Klint Å, Tryggvadóttir L, Gislum M, Engholm G, Bray F, . Trends in the survival of patients diagnosed with malignant neoplasms of lymphoid, haematopoietic, and related tissues in the Nordic countries 1964–2003 followed up to the end of 2006. Acta Oncol 2010;49:694–712.
- Storm HH, Kejs AMT, Engholm G, Tryggvadóttir L, Klint Å, Bray F, . Trends in the overall survival of cancer patients diagnosed 1964–2003 in the Nordic countries followed up to the end of 2006; the importance of case-mix. Acta Oncol 2004;49:713–24.
- Brenner H, Rachet B. Hybrid analysis for up-to-date long-term survival rates in cancer registries with delayed recording of incident cases. Eur J Cancer 2004;40:2494–501.
- Corazziari I, Quinn M, Capocaccia R. Standard cancer patient population for age standardising survival ratios. Eur J Cancer 2004;40:2307–16.
- Cleveland WS. Robust locally weighted regression and smoothing scatterplots. JASA 1979;74:829–36.
- Iversen LH, Nørgaard M, Jacobsen J, Laurberg S, Sørensen HT. The impact of comorbidity on the survival of Danish colorectal cancer patients from 1995–2006: A population-based study. Dis Colon Rectum 2009;52:71–8.
- Lund L, Borre M, Jacobsen J, Sørensen HT, Nørgaard M. Impact of comorbidity on survival of Danish prostate cancer patients, 1995–2006: A population-based cohort study. Urology 2008;72:1258–62.
- Tetsche MS, Nørgaard M, Jacobsen J, Wogelius P, Sørensen HT. Comorbidity and ovarian cancer survival in Denmark, 1995–2005: A population-based cohort study. Int J Gynecol Cancer 2008;18:421–7.
- Jullumstrø E, Kollind M, Lydersen S, Edna TH. Diabetes mellitus and outcomes of colorectal cancer. Acta Oncol 2009;48:361–7.
- Tønnesen H, Nielsen PR, Lauritzen JB, Møller AM. Smoking and alcohol intervention before surgery: Evidence for best practice. Br J Anaesth 2009;102:297–306.
- Janssen-Heijnen ML, Maas HA, Houterman S, Lemmens VE, Rutten HJ, Coebergh JW. Comorbidity in older surgical cancer patients: Influence on patient care and outcome. Eur J Cancer 2007;43:2179–93.
- OECD database. [cited 2009 Nov 3]. Available from: http://titania.sourceoecd.org/vl=726524/cl=36/nw=1/rpsv/home.htm.
- Mørck HI, Linde J, Agner E, Hein HO, Gyntelberg F, Nielsen PE. [Tobacco consumption and smoking patterns in the Nordic countries] (in Danish). Nordisk Medicin 1982;97:134–46.
- Dreyer L, Winther JF, Pukkala E, Andersen A. Avoidable cancers in the Nordic countries. Tobacco smoking. APMIS 1997;105(Suppl 76):9–47.
- Pedersen E, Magnus K, Mork T, Hougen A, Bjelke E, Hakama M, . Lung cancer in Finland and Norway. An epidemiological study. APMIS 1969;(Suppl 199):1–74.
- Gray N. The ethics of policies for the prevention of tobacco disease. Acta Oncol 2004;43:8–10.
- Lambe M. Tobacco control, ethics and Swedish snus. Acta Oncol 2004;43:3–4.
- Giljam H, Galanti MR. Role of ‘snus’ (oral moist snuff) in smoking cessation and smoking reduction in Sweden. Addiction 2003;98:1183–9.
- . Nordic Council of MinistersHakala K, Waller M. Nordic tobacco control. Towards smokefree societies. Copenhagen; 2003.
- Dreyer L, Winther JF, Andersen A, Pukkala E. Avoidable cancers in the Nordic countries. Alcohol consumption. APMIS 1997;105(Suppl 76):48–67.
- Engholm G, Kejs AM, Brewster DH, Gaard M, Holmberg L, Hartley R, . Colorectal cancer survival in the Nordic countries and the United Kingdom: Excess mortality risk analysis of 5 year relative period survival in the period 1999 to 2000. Int J Cancer 2007;121:1115–22.
- Boyle P, Autier P, Bartelink H, Baselga J, Boffetta P, Burn J, . European Code Against Cancer and scientific justification: third version. Ann Oncol 2003;14:973–1005.
- Bretthauer M, Ekbom A, Malila N, Stefansson T, Fischer A, Hoff G, . [Politics and science in colorectal cancer screening] (in Danish). Ugeskr Laeger 2006;168:2563–5.
- Hakama M, Hoff G, Kronborg O, Påhlman L. Screening for colorectal cancer. Acta Oncol 2005;44:425–39.
- Møller B, Fekjaer H, Hakulinen T, Tryggvadóttir L, Storm HH, Talbäck M, . Prediction of cancer incidence in the Nordic countries up to the year 2020. Eur J Cancer Prev 2002;11(Suppl 1):S1–96.
