Abstract
Background. The levels of the soluble urokinase plasminogen activator receptor (suPAR) in blood have been shown to correlate with prognosis in various cancers. Plasma levels of the combined suPAR forms have previously shown to be a strong prognostic marker in the present cohort of CRC patients and could potentially identify high-risk patients among those with early stage disease. In order to investigate whether the individual suPAR forms are stronger prognostic markers than the combined amount we measured the different uPAR forms in serum from the same cohort and evaluated their prognostic significance. Material and methods. The different suPAR forms were measured in serum preoperatively collected from 518 patients. Patients were followed up to nine years (median 7.9 years) and the primary endpoint was overall survival. The different suPAR forms were measured using Time Resolved Fluorescence Immunoassays(TR-FIAs): Intact, suPAR(I–III) by TR-FIA 1; intact and cleaved, suPAR(I–III)+(II–III) by TR-FIA 2; and liberated uPAR(I) by TR-FIA 3. Results. All three uPAR variants demonstrated prognostic significance when evaluated individually. In a multivariable analysis suPAR(I–III)+(II–III) and the liberated uPAR(I) were shown to be independent markers of prognosis (HR=1.74, CI:1.33–2.26; p <0.0001 and HR=1.32; CI:1.02–1.71; p=0.036 respectively), and independent of the clinical baseline variables: age, gender, tumor stage and localization. Conclusion. This study demonstrated that suPAR(I–III)+(II–III) and the liberated uPAR(I) in serum are independent prognostic markers in CRC.
Colorectal cancer (CRC) has an estimated individual lifetime risk of 5% in developed countries. Worldwide CRC accounts for around 1 million new cases per year, and in Europe CRC is the second most prevalent cancer and the second most important in relation to cancer specific death [Citation1]. Treatment of primary colon cancer (CC) is resection of the tumor followed by adjuvant chemotherapy according to histo-pathological tumor stage. For rectal cancer (RC) multimodal approaches have been introduced based on tumor staging by magnetic resonance scanning [Citation2].
Clinical outcome in CRC patients relates to the stage of disease at the time of diagnosis. The postoperative histo-pathological tumor staging based on the tumor, node, metastasis (TNM) system, is presently the strongest predictor of patient outcome and consequently an important tool in patient management following primary tumor resection [Citation3]. However, the TNM staging system does not offer a precise prediction of prognosis for the individual patient. To improve the staging of CRC patients – and thereby the treatment opportunities of these patients – several tumor markers have been proposed as potential candidates for prognostic evaluation in CRC, but the number of markers that actually have proven clinically useful is currently limited [Citation4].
The plasminogen activation (PA) system is involved in tissue remodeling processes, including tumor growth, invasion and metastasis [Citation5–7]. One of the components, the urokinase plasminogen activator receptor (uPAR), has attracted specific interest, as it has been shown to have prognostic impact when determined in both tissue extracts and plasma [Citation8,Citation9,Citation13–15] uPAR is a three-domain glycolipid-anchored protein localizing uPA and thereby plasminogen activation to the cell surface [Citation5]. In addition uPA can cleave uPAR in the linker region between domain I and II, liberating domain I, uPAR(I) and thus inactivating the binding potential of uPAR. The intact glycolipid anchored uPAR, uPAR (I–III), and the cleaved form, uPAR(II–III), can be shed from the cell surface and three soluble uPAR forms, suPAR(I–III), suPAR(II–III) and uPAR(I) are found in different body fluids [Citation14]. The levels of the cleaved uPAR forms both in tumor tissue and in blood reflect the uPA-activity and could therefore potentially be stronger prognostic markers in cancer than the combined amount of uPAR forms [Citation16]. In order to test this, assays measuring the different uPAR forms have been designed. Using these on tumor extracts from non-small cell lung cancer patients the liberated uPAR(I) was found to be a stronger prognostic marker than the combined amount of uPAR forms [Citation17].
The present retrospective cohort study was undertaken to evaluate whether any of the individual uPAR forms determined by the new TR-FIA platforms have improved prognostic value in patients with CRC compared with the value of combined suPAR forms determined by the ELISA platform.
Material and methods
Subjects
From April 1991 through August 1993, patients were included in this multicenter cohort study conducted at Danish hospitals. This study is a prospective placebo-controlled clinical trial studying the effect of treatment with the histamine-II receptor antagonist (Ranitidine) on long-term survival in CRC patients [Citation18]. Patients eligible for inclusion were (18 years or older) undergoing elective resection for histo-pathologically verified primary CRC. Subjects diagnosed with severe concurrent diseases (e.g. other cancers or infectious diseases) and subjects unable to give informed consent were not included in the study. The Regional Ethics Committee and the Danish Data Protection Agency approved the study, and 823 patients were included following informed consent according to the Helsinki II Declaration. Preoperatively collected serum samples were available from 518 of the 823 patients. The primary endpoint was overall survival. The cohort was followed until 2000, resulting in a median follow-up time of 7.9 years (range, 6.5–9.1 years) and 345 events. None of the included patients received adjuvant treatment. The clinical parameters evaluated in this study were: gender; age; localization of tumor; Dukes’ stage; and carcino embryonic antigen (CEA). Since limited amounts of serum were available, all uPAR forms could only be measured in samples from 483 patients, and only these were included in the multivariable analysis. Details are given in .
Table 1. Descriptive statistics shown together with medians and ranges for the different uPAR forms.
Sampling
All samples were collected preoperatively on the day of operation from fasting, supine patients just before skin incision. Serum samples were collected in endotoxin free silicon tubes without additives (Vacutainer® Becton-Dickinson, Mountain View, CA, USA). Following collection samples were left to cloth at room temperature for 1 hour and then spun for 10 minutes at 2 500 g and 4°C. Serum was collected and stored at 280°C until analyzed.
Immunoassay
Measurements of the uPAR variants were performed using three Time Resolved Fluorescence Immunoassays(TR-FIAs); TR-FIA 1 measuring suPAR(I–III), TR-FIA 2 measuring suPAR(I–III)+(II–III), and TR-FIA 3 measuring liberated domain I, uPAR(I) [Citation17]. Their validation in serum (in 1:5 dilution) has previously been described [Citation21]. Samples were read in the Fluostar Galaxy fluorometer with excitation set at 405 nm and emission read at 615 nm with a 400 μs delay and a 400 μs acquisition window.
Statistics
The SAS software package (version 9.1, SAS Institute, Cary, NC) was used for data management and statistical calculations. The uPAR concentrations are presented by the median and range. Hypothesis tests on localization, stage and gender were done using the Wilcoxon rank sum test. Associations between variables were determined by the Spearman rank correlation coefficients. Survival probabilities were estimated using the Kaplan-Meier method for each of the uPAR forms grouped by the respective tertiles, except uPAR(I) which was dichotomized by the limit of quantification (LOQ=20.2 fmol/ml). The Cox proportional hazards model was used for multivariable analysis, and included the covariates: disease stage, localization of tumor, age, gender and the uPAR-forms. suPAR(I–III) and suPAR(I–III)+(II–III) were log transformed (base 2) and uPAR(I) was dichotomized using the limit of quantification as the cut-point. The functional form of the continuous covariates and the proportional hazards assumption were verified using martingale and Schoenfeld residuals. The assumption of proportional hazards was verified graphically where applicable. P-values below 0.05 were considered significant.
Results
Patient characteristics including gender, Dukes’ stage of disease and tumor localization are given in , including the number of events and levels of the different uPAR forms.
Association between the different uPAR forms and other variables
Correlation analyses showed strong correlations between suPAR(I–III) and suPAR(I–III)+(II–III)(r=0.73; p <0.0001) whereas weaker – but still significant – correlations were observed between uPAR(I) and suPAR(I–III) as well as suPAR(I–III)+ (II–III) (r=0.48; p< 0.0001 and r=0.34; p< 0.0001 respectively). All uPAR forms were weakly positive but significantly correlated with patient age (data not shown).
uPAR level and gender, tumor stage and localization
Significant difference levels were shown between Dukes’ stages for all three markers (Wilcoxon rank sum p <0.0001). Furthermore, significantly higher concentrations were observed for all three markers in patients with CC compared to RC patients (p≤0.03). For the intact form, suPAR(I–III), a significant difference was seen for gender (p =0.007), whereas this was not the case for suPAR(I–III)+(II–III) and uPAR(I) ().
Levels of uPAR variants and overall survival
uPAR(I). The group of patients with uPAR(I) levels above the level of quantification (LOQ) had a significantly poorer survival compared to those with levels below (HR =2.04 CI:1.6–2.6; p <0.0001). The groups had a median survival of 16 versus 48 months respectively (). Patients diagnosed with Dukes’ B tumors and with circulating uPAR(I) levels above the LOQ had a significantly poorer survival than those with uPAR(I) levels below LOQ (). Univariable analysis of uPAR(I) scored dichotomized by the LOQ is shown in .
Figure 1. Kaplan-Meier plots illustrating the estimates of survival probabilities for CRC patients stratified by the levels of the uPAR forms. The upper panel shows the survival estimates for uPAR(I) (a) dichotomized by the LOQ (20.2 fmol/ml). The two lower panels show the survival estimates for suPAR(I–III) (b) and suPAR(I–III)+(II–III)(c) in three groups using the tertiles as cut points. The tertiles for suPAR(I–III) were 32.2 and 46.4 fmol/ml, and 90.3 and 117.9 fmol/ml for suPAR(I–III)+(II–III). The p-values shown are for the log rank statistic. The number of patients at risk at 0, 24, 48 and 72 months in each strata are shown below each plot with the number of events (deaths) shown to the left.
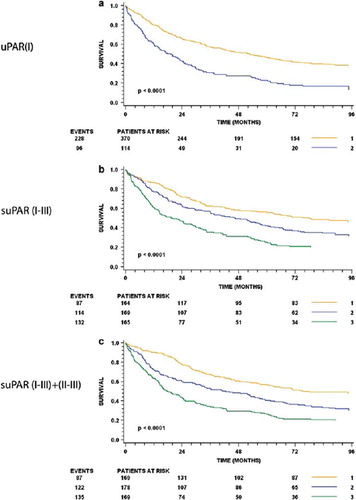
Figure 2. Preoperative serum levels of uPAR(I), suPAR(I–III) and suPAR(I–III)+(II–III) and overall survival for patients with Dukes’ B and Dukes’ C cancer. The upper panel shows the Kaplan-Meier estimates stratified by uPAR(I) (a and b), the middle panel shows the estimates for suPAR(I–III) (c and d) and the lower panel shows the estimates for suPAR(I–III)+(II–III) (e and f). The respective tertiles were used as cut-points. The p-values shown are for the log rank statistic. The number of patients at risk at 0, 24, 48 and 72 months in each strata are shown below each plot with the number of events (deaths) shown to the left.
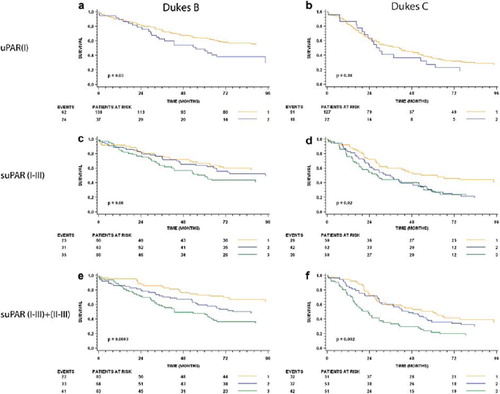
Table 2. Uni- and multivariable regression analyses in 483 colorectal cancer patients.
suPAR(I–III)+(II–III). All samples contained measurable amounts of suPAR(I–III)+(II–III). When patients were grouped by tertiles (90.27 fmol/ml and 117.92 fmol/ml) survival probabilities were shown to be significantly different. Patients with the highest levels of suPAR(I–III)+(II–III) had a median survival of 17 months; patients with the intermediate uPAR(I–III)+(II–III) levels 4.1 years; and patients with the lowest suPAR(I–III)+(II–III) levels 6.5 years (). Similar results were seen for subgroup analyses of Dukes B and C ( and ). In a univariable analysis, suPAR(I–III)+(II–III) scored as a log transformed continuous variable was shown to significantly predict prognosis in this patient cohort (HR=2.51 CI:2.02–3.12; p <0.0001) ().
Intact suPAR. All samples also contained measurable amounts of intact suPAR. Kaplan-Meier estimates of survival probabilities for patients grouped by tertiles (32.2 fmol/ml and 46.6 fmol/ml) demonstrated significance with a median survival of 1.6 years for patients with the highest levels of intact suPAR; 2.1 years for those with intermediate levels and 6.3 years for patients with the lowest levels (). Similarly significant differences were observed, for analyses of Dukes B and C ( and ). In a univariable analysis suPAR(I–III) was shown to significantly predict prognosis in this patient cohort (HR=1.86; CI:1.55–2.22; p<0.0001) ().
Multivariable analysis
A multivariable analysis including all three markers and clinical baseline variables was performed and followed by a stepwise backwards selection. Following these analyses, suPAR(I–III)+(II–III) and uPAR(I), were shown to be independent markers of prognosis in addition to tumor stage, localization, age and gender. Results of the multivariable analyses are shown in .
Univariable analysis of CEA demonstrated that CEA was a significant predictor of overall survival (HR=1.97; CI:1.59–2.44; p<0.0001). When included in the multivariable analysis CEA showed to be non-significant (p=0.49) (). Addition of Ranitidine treatment was not significant (data not shown).
Discussion
The results of the present study showed that the specific suPAR forms in serum had independent prognostic value in patients with CRC; high levels were related to poor prognosis and low levels to good prognosis.
Levels of suPAR in EDTA plasma samples have previously been determined in the present patient cohort [Citation15], using an ELISA platform quantifying the combined amounts of free and uPA occupied suPAR(I–III) and suPAR(II–III). The results showed that the preoperative plasma level of suPAR independently predicted survival in CRC patients; high levels were associated with poor prognosis and low levels with good prognosis. Comparison of the median level of suPAR measured in EDTA plasma by the ELISA in the former study and the levels of suPAR(I–III)+(II–III) measured in serum by the TR-FIA2 in the present study showed that the levels were higher in serum than in plasma. However, we found that the ELISA and TR-FIA 2 suPAR measurements were strongly correlated (r =0.80; p< 0.0001).
Similar results have previously been shown in samples from patients with non-small cell lung cancer [Citation20]. Degranulation of cells and accumulation of proteins stored in granules during coagulation in blood for serum may explain the differences with higher levels of suPAR in serum than in plasma.
The results of the present study also showed that preoperative levels of the different suPAR forms were significantly associated with tumor stage and localization and were weakly correlated with patient age. suPAR levels were different when comparing tumor localization, since colon cancers showed significantly higher uPAR levels than rectal cancers. This was also the case when the analysis was stratified by tumor stage and could not be explained by colon tumors being more advanced than rectal tumors. These findings are corresponding to the findings in a previous study analyzing samples from this same patient cohort [Citation15].
In a univariable analysis the individual uPAR forms determined in the present study showed prognostics value; high levels were associated with poor prognosis and low levels with good prognosis In the subsequent multivariable analysis including gender, age, tumor stage, localization and the different uPAR forms, the levels of suPAR(I–III)+(II–III) and uPAR(I) were shown to be independent predictors of overall survival. Thus, the results seen for uPAR(I) are similar to those previously shown in patients with ovarian and non-small cell lung cancer [Citation19,Citation21]. The different uPAR forms have been evaluated in other studies of cancer patients [Citation17,Citation21,Citation23]. The fact that uPAR(I–III)+(II–III) is retained as the most significant prognostic marker in this study, supports the results of the study by Stephens [Citation15]. The fact of uPAR(I) being less significant as a prognostic marker compared to suPAR(I–III)+(II–III), could be explained by the fact that relatively few individuals have uPAR(I) above the LOQ. Whereas all patients had measurable suPAR(I–III)+(II–III) thereby increasing statistical power to detect differences.
Dukes’ B patients had significant differences in overall survival for low and high levels of uPAR(I). Most interestingly 65% of the patients with measurable uPAR(I) died during follow-up in this patient group. This finding is of specific interest since patients presenting with Dukes’ B (=stage II) cancers are presently not candidates for standard adjuvant therapy, unless they have high-risk features (i.e. obstruction, perforation, inadequate lymph node sampling or T4 disease) [Citation22]. It should however be noted that the tumor classification used in the present study is not comparable with the current practise, and conclusions should be drawn with caution. According to these results further clinical studies evaluating uPAR(I) in Stage II patients and the possible benefit of uPAR(I) guided selection to adjuvant treatment in these patients would be of great interest. The results of the present study support a future application of different uPAR forms in the clinical setting. Primarily as a tool for improved staging of CRC patients, which would allow for an optimized treatment selection in this group of patients.
Acknowledgements
The excellent technical assistance of Ruth Petersson is gratefully acknowledged. The study was supported by: European Community's FP7/2007-2011 grant agreement n°201279, The Kornerup Fund, The Aase and Ejnar Danielsen Fund, The Aage and Johanne Louis-Hansen Fund, The Walter and O. Kristiane Christensen Fund, The Sophus and Astrid Jacobsen Fund, The Arvid Nilsson Fund, The Glunz and Jensen Fund, The Friedrich and Else Boehm Fund, The Agnes and Poul Friis Fund, The Eva and Henry Fraenkel Fund, The Hartmann Bros. Fund, The Willy and Ingeborg Reinhard Fund, The Katrine and Vigo Skovgaard Fund, The Oda and Hans Svenningsen Fund, The Einar Willumsen Fund, and The Danish Cancer Society (Hans Jørgen Nielsen is Kornerup Professor of Surgical Oncology).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ferlay J, Autier P, Boniol M, Heanue M, Colombet M, Boyle P. Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol 2007;18:581–92.
- Smith N, Brown G. Preoperative staging of rectal cancer. Acta Oncol 2008;47:20–31.
- Compton CC, Greene FL. The staging of colorectal cancer: 2004 and beyond. CA Cancer J Clin 2004;54:295–308.
- Sturgeon MC, Duffy MJ, Stenman UH, Lilja H, Brünner N, Chan DW. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines for use of tumor markers in testicular, prostate, colorectal, breast, and ovarian cancers. Clin Chem 2008;54:e11–e79.
- Andreasen PA, Egelund R, Petersen HH. The plasminogen activation system in tumor growth, invasion, and metastasis. Cell Mol Life Sci 2000;57:25–40.
- Werb Z. ECM and cell surface proteolysis: Regulating cellular ecology. Cell 1997;91:439–42.
- Irigoyen JP, Munoz-Canoves P, Montero L, Koziczak M, Nagamine Y. The plasminogen activator system: Biology and regulation. Cell Mol Life Sci 1999;56:104–32.
- Ganesh SJ, Sier C, Heerding M, Griffioen G, Lamers C, Verspaget H. Urokinase receptor and colorectal cancer survival. Lancet 1994;344(8919):401–2.
- Pedersen H, Brunner N, Francis D, Osterlind K, Ronne E, Hansen HH, . Prognostic impact of urokinase, urokinase receptor, and type 1 plasminogen activator inhibitor in squamous and large cell lung cancer tissue. Cancer Res 1994;54: 4671–5.
- Blasi F, Carmeliet P. uPAR: A versatile signalling orchestrator. Nat Rev Mol Cell Biol 2002;3:932–43.
- Ohtani H, Pyke C, Dano K, Nagura H. Expression of urokinase receptor in various stromal-cell populations in human colon cancer: Immunoelectron microscopical analysis. Int J Cancer 1995;62:691–6.
- Illemann M, Bird N, Majeed A, Laerum OD, Lund LR, Dano K, . Two distinct expression patterns of urokinase, urokinase receptor and plasminogen activator inhibitor-1 in colon cancer liver metastases. Int J Cancer 2009;124: 1860–70.
- Konno H, Abe J, Kaneko T, Baba M, Shoji A, Sunayama K, . Urokinase receptor and vascular endothelial growth factor are synergistically associated with the liver metastasis of colorectal cancer. Jpn J Cancer Res 2001;92:516–23.
- Hoyer-Hansen G, Lund IK. Urokinase receptor variants in tissue and body fluids. Adv Clin Chem 2007;44:65–102.
- Stephens RW, Nielsen HJ, Christensen IJ, Thorlacius-Ussing O, Sorensen S, Dano K, . Plasma urokinase receptor levels in patients with colorectal cancer: Relationship to prognosis. J Natl Cancer Inst 1999;91:869–74.
- Piironen T, Laursen B, Pass J, List K, Gardsvoll H, Ploug M, . Specific immunoassays for detection of intact and cleaved forms of the urokinase receptor. Clin Chem 2004;50:2059–68.
- Almasi CE, Hoyer-Hansen G, Christensen IJ, Dano K, Pappot H. Prognostic impact of liberated domain I of the urokinase plasminogen activator receptor in squamous cell lung cancer tissue. Lung Cancer 2005;48:349–55.
- Nielsen HJ, Christensen IJ, Moesgaard F, Kehlet H. Ranitidine as adjuvant treatment in colorectal cancer. Br J Surg 2002;89:1416–22.
- Lomholt AF, Hoyer-Hansen G, Nielsen HJ, Christensen IJ. Intact and cleaved forms of the urokinase receptor enhance discrimination of cancer from non-malignant conditions in patients presenting with symptoms related to colorectal cancer. Br J Cancer 2009;101:992–7.
- Almasi CE, Hoyer-Hansen G, Christensen IJ, Pappot H. Prognostic significance of urokinase plasminogen activator receptor and its cleaved forms in blood from patients with non-small cell lung cancer. APMIS 2009;117:755–61.
- Henic E, Borgfeldt C, Christensen IJ, Casslen B, Hoyer-Hansen G. Cleaved forms of the urokinase plasminogen activator receptor in plasma have diagnostic potential and predict postoperative survival in patients with ovarian cancer. Clin Cancer Res 2008;14:5785–93.
- Figueredo A, Coombes ME, Mukherjee S. Adjuvant therapy for completely resected stage II colon cancer. Cochrane Database Syst Rev 2008;3:CD005390.
- Piironen T, Haese A, Huland H, Steuber T, Christensen IJ, Brunner N, . Enhanced discrimination of benign from malignant prostatic disease by selective measurements of cleaved forms of urokinase receptor in serum. Clin Chem 2006;52:838–44.
