To the Editor,
Gemcitabine is a nucleoside analogue which was first approved in 1996 for the treatment of locally advanced or metastatic pancreatic carcinoma. It is currently used for the treatment of a wide range of malignancies. Primary side effects are myelosuppression, mild liver function abnormalities and flu-like symptoms. A rare, but serious complication of gemcitabine treatment is thrombotic microangiopathy (TMA).
TMA is a disorder characterized by generalized microvascular occlusion by platelet thrombi, leading to organ damage particularly in the brain, kidneys and heart. Injury to the endothelial cell is the central and likely inciting factor in the sequence of events leading to TMA. Classically it presents with Coombs negative hemolytic anemia, thrombocytopenia, fever, renal insufficiency and neurological symptoms. Hypertension and pulmonary symptoms can also occur. In the spectrum of clinical presentations of TMA a distinction is made between thrombotic thrombocytopenic purpura (TTP), with a predominance of neurological symptoms, and the hemolytic-uremic syndrome (HUS), with predominantly renal insufficiency. Severe deficiency of plasma ADAMTS-13 activity is more specific for TTP [Citation1,Citation2]. The distinction between TTP and HUS is, however, not always clear. In the present article we will therefore refer to the syndrome as TMA.
It is very rare for TMA to develop as a complication of gemcitabine treatment already at low cumulative doses or after short treatment duration. In this article we present a patient who developed TMA after a single dose of gemcitabine as fourth-line palliative treatment for metastasized breast carcinoma.
Case report
A 53-year-old woman was admitted to the Medical Oncology ward of the Radboud University Nijmegen Medical Centre with gait problems and visual abnormalities. Seven years earlier she was diagnosed with grade 2, estrogen receptor and progesterone receptor positive, HER2 negative pT2N1(mi) ductal breast carcinoma, for which a mastectomy was performed. A year later metastases in the bones were noted for which she was treated with radiotherapy and two lines of palliative hormonal therapy. During the second-line hormonal therapy hepatic metastases developed and the patient was treated with three lines of chemotherapy and another line of hormonal therapy, but progressive disease was noted after each therapy. Therefore fourth-line palliative chemotherapy with gemcitabine (1250 mg/m2, day 1 and 8, in each three-week cycle) was initiated.
A few days after the first dose of the first cycle of gemcitabine the patient developed fever and therefore the second dose was postponed. Ten days after the first dose the patient presented with drowsiness, abnormal gait and a blurred vision. There were no symptoms of increased intracranial pressure. On physical examination hypertension and jaundice was noted, but no hepatosplenomegaly or focal neurological abnormalities. Eight days after admission she suddenly developed a right-sided hemiparesis and nystagmus. Laboratory examination revealed hypercalcemia, hyperbilirubinemia, liver enzyme abnormalities, high LDH and a Coombs negative hemolytic anemia with a decreased haptoglobin level (). A blood smear showed schistocytes, consistent with microangiopathy. The ADAMTS-13 activity was not determined. There was no thrombocytopenia or renal insufficiency and no signs of diffuse intravascular coagulation. Extensive further examination was performed and hypercalcemia, a primary opthalmologic problem, diffuse intravasal coagulation and compression of the biliary tract was ruled out. An MRI did not reveal intracranial metastases or ischemia, but increased frontal signal intensity was observed on both sides, which however was not specific for posterior reversible encephalopathy syndrome (PRES). Lumbar puncture did not reveal infectious meningitis or malignant cells. As the combination of the microangiopathic hemolytic anemia and neurological symptoms made TMA the most likely diagnosis, treatment with corticosteroids was started. Nevertheless the patient's general condition deteriorated further and because of her very poor prognosis plasmapheresis was not performed. The patient died 28 days after the administration of one dose of gemcitabine. Autopsy was performed and showed widespread microangiopathy in the cerebrum and cerebellum, kidneys, lungs, liver, and adrenal glands, with widespread bleeding (). There were numerous hemorrhagic infarcts in the cerebrum due to microthrombi (). The liver and kidneys showed changes secondary to shock with zone 3 hepatocellular necrosis and acute tubular necrosis, respectively. Widespread metastases were observed in the liver, left lung, ovaries, peritoneum, and bones with high osteoclastic activity around the bone trabeculae and extramedullary hematopoiesis in the spleen was noted.
Figure 1. Microscopic image of the kidney with microthrombi in capillaries of a glomerulus. Haematoxylin and eosin stain (200 × magnification).
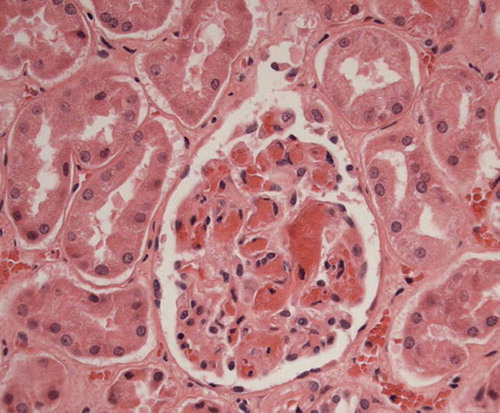
Figure 2. Macroscopy of the central nervous system with hemorrhages. (a) Dorsal view of the cerebrum and cerebellum with multiple, petechial and somewhat larger, partly confluent hemorrhages in cerebral cortex of occipital lobes. (b) Cerebellar hemispheres on cut surface with multiple, petechial and somewhat larger, partly confluent hemorrhages.
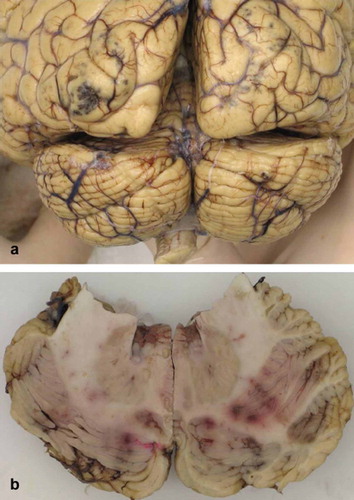
Table I. Laboratory examination of the patient.
Discussion
Gemcitabine-associated TMA (GTMA) was first described by Casper et al. in 1994 in a phase II trial in patients with pancreatic carcinoma treated with gemcitabine. In this group of 44 patients one patient developed hemolytic-uremic syndrome (HUS) [Citation3]. After this initial report several other studies reported the occurrence of GTMA, with different incidences (summarized in ). Fung et al. reported an incidence of 0.015% (12 cases among 78 800 patients) of gemcitabine-associated HUS [Citation4]. Humphreys et al. found an incidence of GTMA of 0.31% (8 cases among 2 586 patients), and Izzedine et al. reported an incidence of 0.4% (3 cases among 706 patients) [Citation5,Citation6]. A relatively high incidence of HUS of 1.4% was reported by Müller et al. after prolonged gemcitabine therapy in patients who had been pretreated with other chemotherapeutic agents (4 of 291 patients) [Citation7]. An equally high incidence was observed by Arnadottir et al., where three patients of 244 patients who had been treated with gemcitabine for six to nine months developed TMA [Citation8].
Table II. Incidence of gemcitabine-associated TMA in different studies.
It is very rare for GTMA to develop already at low cumulative doses or after short treatment duration. It mainly occurs at cumulative doses above 20.000 mg/m2, when the number of doses exceeds 18 or when the treatment duration is above seven months [Citation9]. De Smet et al., however, reported a case of TTP five days after a single dose of gemcitabine in a patient with pancreatic carcinoma who had not received other chemotherapy before [Citation10]. Also, Saif et al. reported a case of a patient with malignant thymoma, who had been pretreated with 20 sessions of mediastinal radiotherapy and developed TMA after the second and third infusion of gemcitabine [Citation11].
The primary treatment of GTMA is discontinuation of the gemcitabine therapy. In case of severe disease corticosteroids, hemodialysis, and plasmapheresis must be considered. Antihypertensive medications are used as needed. In a review of 39 patients developing GTMA Zupancic et al. reported a TMA-associated mortality of 15% [Citation9].
Besides gemcitabine, TMA is described as a complication of other chemotherapeutic agents as well, most frequently after mitomycine and cisplatin [Citation9]. The mechanisms by which drugs cause TMA are not known. The two main proposed mechanisms are an immune-mediated or a direct toxic effect on endothelium [Citation12,Citation13]. Many drug related TMA cases are not associated with severe ADAMTS-13 deficiency or with ADAMTS-13–inhibiting antibodies [Citation13,Citation14]. In case of cancer-induced TMA, Blot et al. report a patient with an undetectable plasma ADAMTS-13 level, which returned to normal levels after clinical improvement of TMA and reduction of tumor markers in response to chemotherapy [Citation15]. However, other studies reported normal or only mildly reduced plasma ADAMTS-13 activity in patients with malignant tumors and TMA [Citation14].
It may be difficult to distinguish between cancer-associated TMA and chemotherapy-associated TMA. In fact, the development of TMA can be a combination of both the malignant disease and the drugs. Thachil hypothesized that chemotherapy can impair the repair of endothelial injury caused by cancer [Citation16]. In our case a sharp rise in bilirubin, LDH and the development of anemia was noticed shortly after the first dose of gemcitabine, which suggests that TMA was induced by the gemcitabine. Furthermore, tumor thrombi or emboli were not seen at autopsy.
In conclusion, we present a patient who developed TMA after a single dose of gemcitabine as fourth-line palliative treatment for metastasized ductal breast carcinoma. This case supports the idea that extensive previous chemotherapy can contribute to the development of TMA. It is important for clinicians to be aware of this potentially lethal complication.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ruggenenti P, Noris MS, Remuzzi G. Thrombotic microangiopathy, haemolytic uremic syndrome, and thrombotic thrombocytopenic purpura. Kidney Int 2001;60:831–46.
- Moake, JL. Thrombotic microangiopathies. N Engl J Med 2002;347:589–600.
- Casper ES, Green MR, Kelsen DP, Heelan RT, Brown TD, Flombaum CD, . Phase II trial of gemcitabine (2,2″-difluorodeoxycytidine) in patients with adenocarcinoma of the pancreas. Invest New Drugs 1994;12:29–34.
- Fung MC, Storniolo AM, Nguyen B, Arning M, Brookfield W, Vigil J. A review of haemolytic uremic syndrome in patients treated with gemcitabine therapy. Cancer 1999;85:2023–32.
- Humphreys BD, Sharman JP, Henderson JM, Clark JW, Marks PW, Rennke HG, . Gemcitabine-associated thrombotic microangiopathy. Cancer 2004;100:2664–70.
- Izzedine H, Isnard-Bagnis C, Launay-Vacher V, Mercadal L, Tostivint I, Rixe O, . Gemcitabine-induced thrombotic microangiopathy: A systematic review. Nephrol Dial Transplant 2006;21:3038–45.
- Müller S, Schütt P, Bojko P, Nowrousian MR, Hense J, Seeber S, . Haemolytic uremic syndrome following prolonged gemcitabine therapy: Report of four cases from a single institution. Ann Hematol 2005;84:110–4.
- Arnadottir M, Benediktsson T, Hrafnkelsson J. The cumulative incidence of gemcitabine-induced thrombotic microangiopathy. Acta Oncol 2007;46:545–6.
- Zupancic M, Shah PC, Shah-Khan F. Gemcitabine-associated thrombotic thrombocytopenic purpura. Lancet Oncol 2007;8:634–41.
- de Smet D, Jochmans K, Neyns B. Development of thrombotic thrombocytopenic purpura after a single dose of gemcitabine. Ann Hematol 2008;87:495–6.
- Saif MW, Xyla V, Makrilia N, Bliziotis I, Syrigos K. Thrombotic microangiopathy associated with gemcitabine: Rare but real. Expert Opin Drug Saf 2009;8:257–60.
- Shah R, Beem E, Sautina L, Zharikov SI, Segal MS. Mitomycin- and calcineurin-associated HUS, endothelial dysfunction and endothelial repair: A new paradigm for the puzzle? Nephrol Dial Transplant 2007;22:617–20.
- Zakarija A, Bennett C. Drug-induced thrombotic microangiopathy. Semin Thromb Hemost 2005;31:681–90.
- Franchini M, Montagnana M, Targher G, Lippi G. Reduced von Willebrand factor-cleaving protease levels in secondary thrombotic microangiopathies and other diseases. Semin Thromb Hemost 2007;33:787–97.
- Blot E, Decaudin D, Veyradier A, Bardier A, Zagame OL, Pouillart P. Cancer-related thrombotic microangiopathy secondary to Von Willebrand factor-cleaving protease deficiency. Thromb Res 2002;106:127–30.
- Thachil, J. Causes of thrombotic thrombocytopenic purpura. Lancet Oncol 2007;8:757–8.
