Abstract
Background. Inhibition of the Insulin-like Growth Factor-1 receptor (IGF-1R) has resulted in extensive anti-tumor effects. Picropdophyllin (PPP, AXL1717) is a small-molecule inhibitor of the IGF-1R without inhibition of closely related receptors including the insulin receptor and has shown extensive effects against a wide range of tumors in animals. PPP is currently tested as an orally administrated single agent treatment in an open-label combined Phase I/II clinical study in advanced cancer patients with solid tumors which progress in spite of several lines of treatment. Patients and methods. The first part (Phase IA) consisted of single day BID dosing every three weeks with consecutive dose escalations. The second part (Phase IB) consists of seven days or longer BID dosing every three weeks, dosing range being 520–700 mg BID. Non-progressing patients could continue treatment within a compassionate use setting. Results and discussion. The present report describes our experience with the four patients with progressive squamous non-small cell lung cancer (NSCLC) that have received treatment with PPP. Despite more than seven months of PPP treatment as third or fourth line treatment, the reported patients did not develop any additional metastases. Furthermore, CT scans as well as 18FDG-Positron Emission Tomography (PET) scans of the patients demonstrated large central necrotic areas, which may suggest tumor response. At the same time, the study drug is so far well tolerated. The phenomenon of necrosis in the tumors suggestive of tumor response has not been reported before in anti-IGF-1R treatment and will be subject to further studies in the present clinical trial.
Insulin-like Growth Factor-1 (IGF-1) is a well-known regulator of cellular metabolism and growth. There is considerable evidence that IGF-1 and the IGF-1 receptor pathway are involved in neoplasia and that many different cancer cells are more dependent on this pathway than corresponding normal cells [Citation1]. Inhibitors of IGF-1R have gained a lot of interest lately but most of the small-molecule inhibitors have affinity to the closely related insulin receptor [Citation2,Citation3]. In addition, IGF-1R inhibitors tested in clinical trials (mainly antibodies) have shown interesting effects when tested in patients with squamous non-small cell lung cancer (NSCLC) in combination with cytotoxic chemotherapy but reports regarding single agent activity of these agents are lacking [Citation2,Citation3]. Recently, members of the cyclolignane family have been identified as potential inhibitors of the tyrosine kinase activity of IGF-1 receptors. Picropodophyllin (PPP), an epimer of podophyllotoxin (PPT), shows particular promise since it demonstrates selectivity for IGF-1R without inhibiting activity of the insulin receptor or other more distantly related receptors [Citation4]. Inhibition with PPP did not interfere with ATP binding in the kinase domain, which instead suggests inhibition at the substrate level. In cell-free experiments PPP efficiently inhibited autophosphorylation of the IGF-1R with an IC50 value of ∼1 nM. In preclinical models, PPP has demonstrated promising anti-tumor activity in several types of malignancies, including multiple myeloma, melanoma and glioblastoma [Citation5,Citation6]. In the present case report, we describe the experience from the four patients with progressive squamous NSCLC participating in the Phase I/II study in which PPP, named AXL1717 in the present study, has been tested.
This is the first report from this ongoing clinical trial describing findings that suggest a possible anti-tumor response in NSCLC patients. Further reports describing the safety, pharmacokinetics and biological effects from the whole study group will be published at the conclusion of the trial.
Patients and methods
Study design
PPP (AXL1717) is presently tested as an orally administrated single agent treatment in an open-label combined Phase I/II clinical study in advanced cancer patients with solid tumors that progress in spite of several lines of treatment. Inclusion criteria are histologically confirmed diagnosis of advanced solid or haematological malignancy not amenable to standard treatment, patients at least 18 years of age, performance status ECOG ≤ 2, pharmacological treatment attempt justified, preserved major organ functions. Exclusion criteria include known malignancy in CNS, performance status ECOG > 2, prior anti-tumor therapy within four weeks of enrolment and life expectancy less than three months. The first part of the clinical study consisted of single day BID dosing every three weeks with consecutive dose escalations. The single dosing part of the study is now complete and patients are presently treated with seven day BID treatment with consecutive dose escalations. Patients are treated for an accumulated total of 28 BID treatment days within the study with an option for non-progressing patients to continue treatment within a compassionate use setting. Study objectives include identifying recommended Phase II dose, pharmacokinetics, safety and biological effects including anti-tumor effects. The protocol was conducted in accordance with Good Clinical Practice guidelines and with the Helsinki Declaration of 1975, as revised in 1983, and was approved by our regional Ethics Review Board. All patients signed written informed consent before enrolment. A total of 24 patients with various solid tumors were by January 30, 2009 included in the study. Patients accepted per protocol were histologically confirmed diagnosis of advanced solid or haematological malignancy not amenable to standard treatment. Patients actually included had the following diagnoses (other than squamous NSCLC): sinonasal undifferentiated carcinoma (SNUC) (1 patient), malignant melanoma (1), esophageal cancer (4), colorectal cancer (4), adenoid cystic carcinoma of the parotid gland (1), chondrosarcoma (1), gastric cancer (2), small cell lung cancer (2), NSCLC adenocarcinoma (1), prostate cancer (1), B-cell lymphoma (1). The present paper reports the experience from the four patients with progressive squamous NSCLC having received extended BID single agent treatment with PPP as a third or fourth line treatment. The reported patients represent four out of five patients with squamous NSCLC so far included in the study; the fifth patient was excluded from the study at an early stage due to poor performance status and was accordingly not evaluable. This patient initially fulfilled the inclusion criteria of the study and received treatment, but the patient deteriorated at an early stage of the study due to disease progression and complications with infection. Relationship with the study drug can not be excluded.
Patient characteristics
Patient 1 is 81 years of age and was diagnosed with squamous non-small cell lung cancer in 2006. During the 30 months since diagnosis she had received two courses of first line chemotherapy (gemcitabine/navelbine) plus radiotherapy, second line therapy with erlotinib as well as four courses of third line (gemcitabine) with stable disease (SD) as best treatment response. At study inclusion she was progressive on CT.
Patient 2 is 84 years of age and was diagnosed with squamous NSCLC in 2005. During the 35 months since diagnosis she had received two courses of first line chemotherapy (carboplatin/navelbine) plus radiotherapy and four courses of second line chemotherapy (gemcitabine) with SD as best treatment response. At study inclusion she was progressive on CT.
Patient 3 is 66 years of age and was diagnosed with squamous NSCLC in 2007. During the 23 months since diagnosis he had received two courses of first line chemotherapy (cisplatin/docetaxel) plus radiotherapy 2–68 Gy with cetuximab concomitantly, as well as second line therapy with pemetrexed with SD as best treatment response. At study inclusion he was progressive on CT.
Patient 4 is 59 years of age and was diagnosed with squamous NSCLC in 2005. During the 45 months since diagnosis he had received six courses of first line chemotherapy (cisplatin/vinorelbine/cetuximab), weekly cetuximab, palliative radiotherapy, second line chemotherapy with three courses of docetaxel as well as third line therapy with erlotinib, with SD as best treatment response. At study inclusion he was progressive on CT.
Results
Treatment
Patient 1 was treated with the initial dose of PPP in September 2008, which was a single-day dose of 160 mg BID. She was subsequently treated every three weeks with minor variations with consecutively increasing single-day doses, eventually receiving her 14th single-day treatment of 2 900 mg BID. The patient was discontinued in July 2009 due to progressive disease after almost ten months of treatment.
Patient 2 was treated with the initial dose of PPP in October 2008, which was a single-day dose of 290 mg BID. She was subsequently treated every three weeks with minor variations with consecutively increasing single-day doses, eventually receiving her tenth single-day treatment of 2 900 mg BID. The patient died after two weeks of hospitalization in May 2009 after seven months of treatment, cause of death being progression of her lung cancer and old age.
Patient 3 was treated with the initial dose of PPP in February 2009, which was a single-day dose of 700 mg BID. He was subsequently treated every three weeks with minor variations with consecutively increasing single-day doses receiving his fifth single-day treatment of 2 200 mg BID in May 2009. He was later treated with 7-day BID treatment with an aim of consecutive dose escalations, starting at a dose of 930 mg BID. A neutropenic event necessitated a dose reduction and he was subsequently treated every four weeks with minor variations, receiving a fourth 7-day treatment of 520 mg BID. The patient demonstrated SD at the end of study treatment, then continuing treatment with 520 mg BID during seven days in 28-day cycles within the compassionate use program. The patient was discontinued in November 2009 due to progressive disease after almost ten months of treatment.
Patient 4 was treated with the initial dose of PPP in March 2009, which was a single-day dose of 930 mg BID. He was subsequently treated every three weeks with minor variations with consecutively increasing single-day doses, receiving a second single-day treatment of 1 240 mg BID. Patient 4 was then treated with 7-day BID treatment with consecutive dose escalations, starting at a dose of 700 mg BID. He was subsequently treated every four weeks with minor variations with consecutively increasing doses receiving a second 7-day treatment of 930 mg BID. He then continued with 14-day treatment of 930mg BID starting in June 2009. The patient demonstrated SD at the end of study treatment, then continuing treatment with 930 mg BID during 14 days in five week cycles within the compassionate use program. The patient is still treated within the compassionate use program after ten months of treatment.
Efficacy
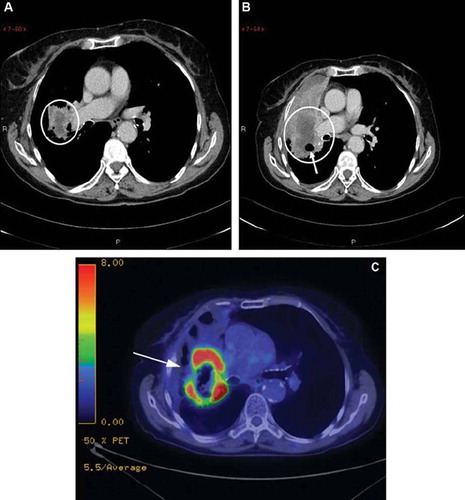
The pre-treatment CT scan of patient 1 from May 22, 2008 showed a primary tumor in the right upper lobe without any known metastasis (). The pre-treatment CT scan also showed clear progression of the disease in comparison to the previous CT scan from January 14, 2008. Patient 1 later had a chest and abdominal CT scan on March 2, 2009, showing that the primary tumor in the right upper lobe had become slightly larger, but was now necrotic, including two air bubbles within the tumor (). No additional tumor manifestations were present compared to the previous CT scan. An 18F-FDG-PET scan from April 2, 2009 showed an uptake of 18F-FDG in the periphery of the tumor with a SUV max of 15.6 (arrow), but not in the central area of necrosis or in the anterior obstructive atelectasis ().
Figure 1. (A) Lung carcinoma in an 81-year-old woman. Transaxial section CT scan from May 22, 2008 obtained at the level of the right pulmonary artery shows a low-density mass adjacent to the right hilus (circle). (B) Image at the same level at ten month follow-up March 2, 2009 shows progress of the mass (circle) with atelectasis ventrally. Note the cavitary air bubble within the mass (arrow). (C) 18F-FDG-PET scan from April 2, 2009 with a pathological uptake of 18F-FDG in the periphery of the tumor (arrow), but not in the central area of necrosis or in the anterior obstructive atelectasis.
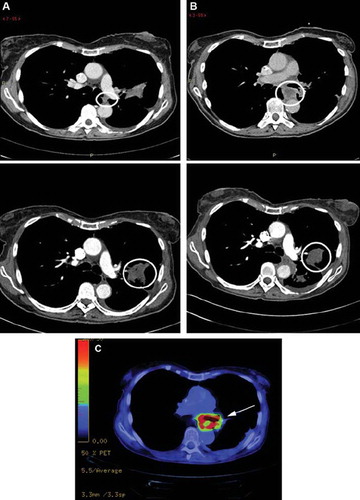
The pre-treatment CT scan of patient 2 from July 3, 2008 showed a progressive primary tumor in the left upper lobe without any known metastasis (); this was compared to a previous CT scan from May 5, 2008. Patient 2 had an on-treatment chest and abdominal CT scan January 18, 2009 showing that the primary tumor in the left upper lobe had slightly increased in volume, but was now more confinable. Furthermore, in the mediastinum ventrally of the descending aortae an expansion was found, 2.5 cm in diameter with contrast enhancement in the periphery and necrotic in the center (), and this expansion was found unchanged on a CT scan from March 18, 2009. An 18F-FDG-PET scan from April 9, 2009 showed a high uptake of 18F-FDG with a SUV max of 15 in the periphery of the expansion in front of descending aortae, whereas the center of the lesion showed low metabolic activity. No pathological uptake was found in the primary tumor in the left upper lobe ().
Figure 2. (A) Transverse CT image from July 3, 2008 in an 84-year-old woman with a tumor in the left upper lobe (lower picture; circle) shows soft tissue mass (upper picture; circle) between the left pulmonary artery and aorta descendens. (B) At three months after the therapy start, January 18, 2009, the primary tumor in the left upper lobe is slightly larger in volume but more confinable (lower picture; circle), whereas the lesion close to aorta descendens has become larger and necrotic (upper picture; circle). (C) 18F-FDG-PET scan from April 9, 2009 with a pathological uptake of 18F-FDG in the periphery of the expansion in front of descending aortae, but a low metabolic activity in the center of the lesion. No pathological uptake was found in the primary tumor in the left upper lobe.
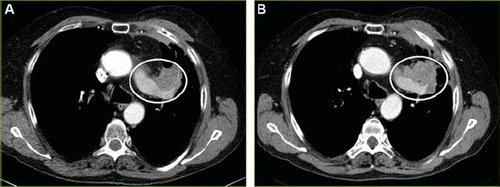
The pre-treatment CT scan of patient 3 from January 7, 2009 showed a progressive primary tumor in the left upper lobe without any known metastasis (); this was compared to a previous CT scan from September 26, 2008. Patient 3 had an on-treatment chest and abdominal CT scan July 22, 2009 showing that the primary tumor in the left upper lobe was unchanged without any additional metastasis. A CT scan at the end of the study, September 23, 2009 demonstrated SD without any additional changes compared to previous CT scan (). He then continued treatment as compassionate use.
Figure 3. (A) Lung carcinoma in a 66-year-old man. Transaxial section CT scan from January 7, 2009 shows a primary tumor in the left upper lobe (circle) without any known metastasis. (B) A CT scan at the end of the study, September 23, 2009, demonstrates stable disease without any additional changes compared to previous CT scan.
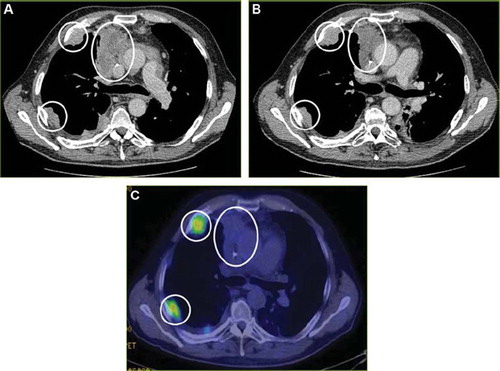
The pre-treatment CT scan of patient 4 from March 17, 2009 showed a progressive primary tumor in the right upper lobe, as well as two metastases in the right thoracic wall, but without any further metastasis (); this was compared to a previous CT scan from January 15, 2009. Patient 4 had an on-treatment chest and abdominal CT scan April 23, 2009 showing that the primary tumor in the right upper lobe was unchanged without any additional metastasis or changes. A CT scan at the end of the study July 21, 2009 () demonstrated SD without any additional changes compared to previous CT scans. An 18F-FDG-PET scan from July 15, 2009 showed no uptake of 18F-FDG in the primary tumor and with a modest uptake in the metastases of the thoracic wall (). The patient then continued treatment as compassionate use.
Figure 4. (A) Transverse CT image from March 17, 2009 in a 59-year-old man shows a primary tumor in the right upper lobe, as well as two metastases in the right thoracic wall (circles), but without any further metastasis. (B) A CT scan after four months of study treatment, July 21, 2009, demonstrates stable disease without any additional lesions. (C) 18F-FDG-PET scan from July 15, 2009 shows no uptake of 18F-FDG in the primary tumor and with a modest uptake in the metastases of the thoracic wall (circles).
Safety
None of the patients included in the single-day BID dosing part of the clinical trial experienced any dose-limiting toxicity. The 7-days BID multi-dosing part of the study is presently ongoing and will be reported when the study has been concluded. The four patients included in the present report have reported good tolerability with the exception of patient 3 who reported a neutropenic event that necessitated a dose reduction from 930 mg to 520 mg BID. Patient 3 was subsequently treated for extended time periods without reporting any further toxicity. All patients have been closely followed with respect to variables involved in the glucose metabolism (blood levels of glucose, insulin and C-peptide). No drug-related changed have to date been reported with respect to these variables suggesting that PPP does not inhibit the insulin receptor.
Discussion
The presently reported patients with squamous cell lung carcinoma have, like the other patients, been treated with good tolerability. In the single-day BID dosing part no dose-limiting toxicity was observed. The neutropenic event observed for patient 3 in the 7-day BID dosing part was a dose-holding toxicity but was manageable, and the patient subsequently continued treatment without further toxicity. The radiological responses are interesting since neither of the patients have metastasized further during treatment. Furthermore, CT scans as well as PET scans on two of the patients after treatment showed that the primary tumor masses displayed necrotic areas which may be suggestive of tumor response. Tumor necrosis/cavitation is an observed phenomenon in tumors of NSCLC. With the current techniques it is very difficult to distinguish between spontaneous and treatment-induced necrosis. However, a lack of necrosis before start of study treatment as well as lack of other clinical signs of progression, including patient deterioration, would argue for a treatment effect, which may be analogous to the treatment effect with cavitation observed for other small-molecule receptor tyrosine kinase inhibitors [Citation7]. Having progressive disease at the start of treatment, all patients show encouraging survival times of seven to ten months and more. In a less selected population of NSCLC patients with progressive disease receiving best supportive care, a median survival time of three months was observed [Citation8]. In the second line setting, median overall survival times ranging between 5.3 to 7.9 months have been reported [Citation9]. The current data are in line with the findings from another Phase I/II clinical trial comparing carboplatin and paclitaxel with and without a monoclonal antibody against IGF-1R, CP-751,871, in stage IIIB and IV NSCLC, showing a higher activity in squamous cell carcinoma histologies [Citation10].
In the present study, we have observed that in parallel to the tumors becoming necrotic, the size of the tumors slightly increased. Similar observations have been made in gastrointestinal stromal tumors (GISTs), where Kobayashi et al. reported a case of recurrent GIST treated with the tyrosine kinase inhibitor imatinib mesylate (Glivec, Novartis), displaying on CT scans an increase of tumor size but a decrease in tumor density three months after initiation of treatment [Citation11]. Thus, paradoxically tumors may enlarge during treatment but, if associated with an overall decrease in tumor enhancement, does not indicate progression. Such enlargements can be due to development of an intra-tumoral hemorrhage, necrosis or to myxoid degeneration [Citation12]. The technique of 18F-FDG-PET may be used to detect both short-term and long-term tumor responses that may not be apparent with CT. On the other hand, even when a patient benefits from treatment, it may take weeks, months, or even years for the tumors to shrink on CT [Citation13].
In conclusion, we describe the activity of the small molecule IGF-1-receptor inhibitor PPP (named AXL1717 in the present study) in four patients with squamous cell lung carcinoma participating in a Phase I/II study. We observe an interesting finding of necrosis in the tumors without any reduction of tumor volume and without progression of disease after at least seven months of treatment. At the same time, the study drug has so far good tolerability. The phenomenon of necrosis in the tumors suggestive of tumor response has not been reported before in anti-IGF-1R treatment and will be subject to further studies in the present clinical trial. The relatively long survival times observed will be further evaluated during the course of the study.
Since single agent activity of inhibitors of IGF-1R have not yet been reported in the public domain with respect to NSCLC, the findings in the present report suggesting the possibility of tumor response may therefore be a novel observation. Further studies are therefore needed to explore the possibility of single agent activity of IGF-1R inhibitors such as PPP.
Acknowledgements
The authors want to thank the patients involved in this study. Research related to this work was conducted without outside funding. Dr Johan Harmenberg is a consultant of Axelar AB och owns options in Axelar AB. Birgitta Ståhl is a consultant of Axelar AB. The other authors report no conflicts of interest.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer 2008;8:915–28.
- Gualberto A, Pollak M. Clinical development of inhibitors of the insulin-like growth factor receptor in oncology. Curr Drug Targets 2009;10:923–36.
- Gualberto A, Pollak M. Emerging role of insulin-like growth factor receptor inhibitors in oncology: Early clinical trial results and future directions. Oncogene 2009;28:3009–21.
- Girnita A, Girnita L, del Prete F, Bartolazzi A, Larsson O, Axelson M. Cyclolignans as inhibitors of the insulin-like growth factor-1 receptor and malignant cell growth. Cancer Res 2004;64:236–42.
- Stromberg T, Ekman S, Girnita L, Dimberg LY, Larsson O, Axelson M, . IGF-1 receptor tyrosine kinase inhibition by the cyclolignan PPP induces G2/M-phase accumulation and apoptosis in multiple myeloma cells. Blood 2006;107:669–78.
- Yin S, Girnita A, Stromberg T, Khan Z, Andersson S, Zheng H, . Targeting the insulin-like growth factor-1 receptor by picropodophyllin as a treatment option for glioblastoma. Neuro Oncol 2010;12:19–27.
- Crabb SJ, Patsios D, Sauerbrei E, Ellis PM, Arnold A, Goss G, . Tumor cavitation: Impact on objective response evaluation in trials of angiogenesis inhibitors in non-small-cell lung cancer. J Clin Oncol 2009;27:404–10.
- Hatzidaki D, Agelaki S, Mavroudis D, Vlachonikolis I, Alegakis A, Georgoulias V; Lung Cancer Group of the Hellenic Oncology Research Group. A retrospective analysis of second-line chemotherapy or best supportive care in patients with advanced-stage non-small-cell lung cancer. Clin Lung Cancer 2006;1:49–55.
- Bedano PM, Hanna N. Salvage therapy in patients with advanced non-small cell lung cancer. J Thorac Oncol 2006;6:582–7.
- Karp DD, Paz-Ares L, Novello S, Haluska P, Garland L, Cardenal F, . High activity of the anti-IGF-IR antibody CP-751,871 in combination with paclitaxel and carboplatin in squamous NSCLC. ASCO Meeting Abstracts 2008;15 (Suppl):8015.
- Kobayashi M, Okamoto K, Nakatani H, Okabayashi T, Namikawa T, Ichikawa K, . Complete remission of recurrent gastrointestinal stromal tumors after treatment with imatinib: Report of a case. Surg Today 2006;36:727–32.
- Hong X, Choi H, Loyer EM, Benjamin RS, Trent JC, Charnsangavej C. Gastrointestinal stromal tumor: Role of CT in diagnosis and in response evaluation and surveillance after treatment with imatinib. Radiographics 2006;26:481–95.
- Van den Abbeele AD. The lessons of GIST-PET and PET/CT: A new paradigm for imaging. Oncologist 2008;13:8–13.
