Abstract
Introduction. In patients with advanced ovarian cancer undergoing preoperative PET/CT, we investigated the prognostic value of SUV in the primary tumor and we evaluated the value of SUV for predicting incomplete primary cytoreduction (macroscopic residual tumor). Material and methods. From September 2004 to August 2007, 201 consecutive patients with a pelvic tumor and a Risk of Malignancy Index (RMI) > 150 based on serum CA-125, ultrasound examinations and menopausal state, underwent PET/CT within two weeks prior to standard surgery/debulking of a pelvic tumor. At two-year follow-up (August 15, 2009) the association between SUV and overall survival/cytoreductive result were analyzed in 60 ovarian cancer patients (58 stage III and two stage IV). Results. At inclusion median age was 62 years (range 35–85 years); 97% (58/60) had a performance status ≤2; 42% (25/60) underwent complete debulking (no macroscopic residual tumor); median SUVmax was 13.5 (range 2.5–39.0). Median follow-up was 30.2 months. At follow-up 57% (34/60) were alive and 43% (26/60) had died from ovarian cancer. SUVmax in patients alive was not statistically different from SUVmax in dead patients (p=0.69), and SUVmax was not correlated with the amount of residual tumor after surgery (p=0.19). Using univariate Cox regression analysis, residual tumor was a significant prognostic variable (p=0.001); SUVmax was not a statistically significant prognostic variable (p=0.86). Discussion. FDG uptake (SUVmax) in the primary tumor of patients with advanced ovarian cancer was not a prognostic variable and the FDG uptake did not predict complete cytoreduction after primary surgery. Future prospective clinical trials will need to clarify if other PET tracers can serve as prognostic variables in ovarian cancer.
In FDG-PET, the FDG uptake in tumors is measured as the standardized uptake value (SUV) [Citation1,Citation2]. Multiple cancer studies indicate that a high SUV in a primary tumor correlates with tumor aggressiveness and poor patient outcome [Citation2–7]. However, no reports exist describing the prognostic value of SUV in primary ovarian cancer.
In ovarian cancer, standard treatment includes primary cytoreductive surgery followed by adjuvant chemotherapy. In theory, ovarian tumors with high SUVs might be more aggressive and patients with such tumors might have a higher risk of undergoing incomplete cytoreduction with macroscopic residual tumor after primary surgery. The value of SUV for predicting residual abdominal tumor after primary surgery has never been described.
The aim of this study was to investigate the prognostic value of SUV in primary ovarian cancer and to evaluate the value of SUV for predicting incomplete primary cytoreduction.
The study was conducted as part of the Danish Pelvic Mass study.
Material and methods
Approval to conduct this study was obtained from the Scientific Ethics Committee in the study area (KF01-227/03 and KF01-143/04).
From September 2004 to August 2007, 201 consecutive patients referred to surgery for a suspected pelvic tumor underwent PET/CT within two weeks prior to standard surgery/debulking. All patients had a Risk of Malignancy Index (RMI) > 150 based on serum cancer antigen 125 (CA-125), ultrasound examinations and menopausal state [Citation8]. Patients were excluded if they were suffering from claustrophobia, severe obesity, known allergy to contrast media, diabetes or other severe medical condition or had a history of previous cancer or borderline tumor.
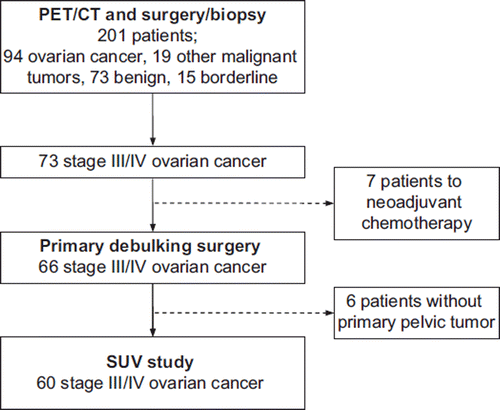
The result of the PET/CT scan was given to the referring surgeon. Patients with a suspicious pelvic mass had an exploratory laparotomy performed by one of the six gynecologic oncologists at our clinic. Of the 201 patients undergoing PET/CT, 94 had ovarian cancer; seven were sent to neoadjuvant chemotherapy and 87 underwent maximal tumor reduction and staging according to FIGO. Complete debulking was defined as no macroscopic tumor left in the pelvis or abdomen at the end of surgery. An experienced pathologist evaluated all operation specimens according to the latest WHO classification (2002). Sixty of the 87 patients undergoing primary debulking surgery had advanced ovarian cancer and a primary pelvic tumor and were eligible for this SUV study ().
PET/CT scanning procedure
PET was performed with an integrated PET/CT (GE Discovery PET/CT, General Electric Medical Systems, Milwaukee, WI). The patient fasted a minimum of six hours before PET acquisition. Sixty minutes after injection of 400 MBq FDG in a cubital vein, the CT scan was performed. The patient was asked to void before scan start. Oral and intravenous contrast agents were given before the CT scan. The CT scan lasted 20 seconds and it was performed with 140 kV and 90–120 mA. Immediately after, the static emissions in 2D acquisition were obtained with 7–8 consecutive frames, each of three minutes’ emission, from the proximal femur to the scalp. The PET acquisition lasted approximately 25 minutes. The CT data were used for attenuation correction of the PET data. Images were reconstructed and stored in 5 mm transaxial, coronal and sagittal slices. The images were fused on the GE eNTEGRA workstation.
Imaging analysis
The same nuclear medicine physician and radiologist evaluated CT and PET images separately and as fused images in consensus.
A pathological uptake was described as focal areas visual in all three planes with the same co-ordinates (x, y, z). Artifactual and physiological soft tissue FDG accumulation was taking into account in the visual interpretation of PET. For each PET study, the SUVmax of the primary pelvic tumor was measured. SUV is a semiquantitative analysis of radiotracer uptake and is defined as the ratio of radiotracer activity per milliliter of tissue to the activity in the injected dose corrected for decay and the patient's body weight [Citation9].
Data analysis
Non-parametric analysis was applied to the data. Validated patient data, data about residual tumor after primary surgery, and the date and cause of death were obtained from the Danish Gynecological Cancer Database. Overall survival was calculated as the interval from primary surgery to death due to ovarian cancer or to August 15, 2009. Using the Mann-Whitney test, SUVmax in patients alive at follow-up was compared with SUVmax in patients dead at follow-up, and SUVmax in patients undergoing complete debulking was compared with SUVmax in patients undergoing incomplete debulking.
The correlation between SUVmax and the amount of residual tumor after surgery was estimated by the Spearman's rank correlation coefficient. The influence of SUVmax and residual tumor on overall survival was explored using univariate Cox regression analysis [Citation10]. Survival curves comparing the survival of patients with SUVmax above the median SUVmax with the survival of patients with SUVmax below the median SUVmax were constructed using the method of Kaplan and Meier [Citation11]. The level of significance was tested using the Log Rank test. P<0.05 was considered the level of significance. The SPSS statistical software system for Windows (SPSS version 18.0, Chicago, IL) was used for the statistical analysis.
Results
Patient characteristics
Sixty patients composed the study population; 58 patients were diagnosed with FIGO stage III; two were diagnosed with FIGO stage IV (one patient had metastases to lymph nodes on the neck, and one patient had liver metastases). Histological diagnoses and stages are listed in . The median age of the 60 patients was 62 years (range 35–85), 58 (97%) had a Gynecologic Oncology Group (GOG) performance status of 2 or better, and the median serum CA125 was 512 U/ml (range 39–4 400 U/ml).
Table I. Histological diagnoses and stages in 60 stage III/IV ovarian cancer patients.
All patients underwent maximal debulking surgery followed by six to eight series of standard chemotherapy with carboplatin and docetaxel. Complete debulking was accomplished in 25 (42%) patients (24 FIGO stage III, and one FIGO stage IV); 36 (60%) underwent cytoreduction with ≤1 cm residual tumor; 44 (73%) underwent cytoreduction with ≤2 cm residual tumor.
Survival analysis
After a median follow-up of 30.2 months, 34 of the 60 patients (57%) were alive and 26 patients (43%) had died from ovarian cancer. The median overall survival of all patients was 30.2 months (range 1.1–58.3 months; quartiles 23.6–42.8 months); the median overall survival of the 34 patients alive at follow-up was 35.9 months (range 23.8–58.3 months; quartiles 28.5–45.4); the median overall survival of patients dead at follow-up was 21.0 months (range 1.1–49.9 months; quartiles 10.9–38.2 months). Of the 25 patients undergoing complete debulking five (20%) had died from ovarian cancer; of the 35 patients undergoing incomplete debulking 21 (60%) had died from ovarian cancer. lists the median SUVmax of all patients, patients alive at follow-up, patients dead at follow-up, patients undergoing complete debulking, and patients undergoing incomplete debulking. At follow-up SUVmax in patients alive was not statistically different from SUVmax in dead patients (p=0.69). SUVmax was not correlated with the amount of residual tumor (p=0.19); SUVmax in patients undergoing complete debulking was not statistically different from SUVmax in patients undergoing incomplete debulking (p=0.38).
Table II. Median SUVmax in 60 stage III/IV ovarian cancer patients, in patients alive/dead at follow-up, and in patients undergoing complete/incomplete debulking.
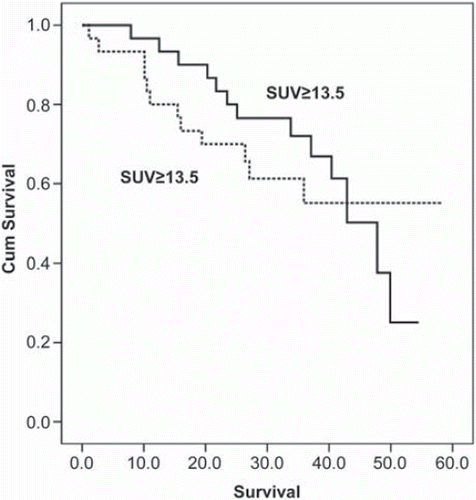
Additionally, SUVmax and residual tumor after primary surgery were evaluated as prognostic variables using univariate Cox regression analysis. Residual tumor was a significant prognostic variable (p=0.001); however, SUVmax was not a statistically significant prognostic variable (p=0.86). The median SUVmax was 13.5. In the survival curve of patients with SUVmax ≥13.5 is compared with the survival curve of patients with SUVmax <13.5; the difference between the curves was not statistically significant (p=0.76).
Discussion
In this study, the FDG uptake (SUVmax) in the primary tumor was not a prognostic variable. The FDG uptake in tumors is influenced by the expression of glucose transport proteins and cytoplasmatic hexokinase activity. Additionally, the FDG uptake is affected by the variability in cellular density, macroscopic and microscopic blood supply, the extent of hypoxic tissue, cellular proliferation, and multiple enzyme systems determining the metabolic activity [Citation12]. These factors also contribute to characterize the tumors’ aggressiveness and malignant potential. This might explain the observed association between high FDG uptake in primary tumors of different cancers and poor patient outcome [Citation2–7].
However, our results show that in stage III/IV ovarian cancer patients, overall survival does not correlate with high FDG uptake in the primary tumor.
In theory, FDG uptake might still have prognostic value in stage I/II ovarian cancer patients or in subgroups of patients with the same tumor histology. In this study, the number of included patients was too small to compare survival of histological subgroups.
Multiple studies have shown that residual tumor after primary surgery is an independent predictor of survival in ovarian cancer patients [Citation13–15]. Researchers have attempted to find clinical and radiological predictors of incomplete cytoreduction; however, no predictor has been located that consistently identify those patients who will undergo incomplete cytoreduction [Citation15–18], and predictors have shown a lack of generalizability when applied to different cohorts of patients [Citation19]. In this study, we investigated the value of FDG for predicting residual tumor after primary surgery (incomplete cytoreduction); however, we found no relation between the FDG uptake (SUVmax) in the primary tumor and incomplete debulking. Consequently, a high FDG uptake in the primary tumor should not be used to withhold ovarian cancer patients from primary surgery and the potential survival benefit of optimal cytoreduction. In primary ovarian cancer, the diagnostic value of FDG-PET and combined FDG-PET/CT is high [Citation9,Citation20,Citation21]. Preliminary data suggest that FDG-PET may be promising for early prediction of response to chemotherapy [Citation22,Citation23]. Additionally, FDG-PET and FDG-PET/CT have proven valuable for detecting recurrent ovarian cancer, especially when CA-125 levels are rising and CT findings are normal or inconclusive [Citation24,Citation25].
Imaging modalities visualizing metabolic pathways provide an opportunity to monitor the metabolism of tumors in vivo and follow the results of therapy. In this study, FDG was not a prognostic variable. Nevertheless, future translational research might find other biomarkers with prognostic value that can be visualized on PET. These biomarkers could potentially alter the treatment course of ovarian cancer patients. However, routine use of new biomarkers or new imaging modalities cannot be advised before the clinical benefit of using these new diagnostic tests have been documented in prospective clinical trials.
In this study, the FDG uptake (SUVmax) in the primary tumor of patients with advanced ovarian cancer was not a prognostic variable and the FDG uptake did not predict complete cytoreduction after primary surgery.
Future prospective clinical trials will need to clarify if other PET tracers can serve as prognostic variables in ovarian cancer.
Before new diagnostic tests are implemented in daily clinical practice, they should undergo careful evaluation in clinical trials using patient related endpoints such as overall survival.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Lucignani G, Paganelli G, Bombardieri E. The use of standardized uptake values for assessing FDG uptake with PET in oncology: A clinical perspective. Nucl Med Commun 2004;25:651–6.
- Kidd EA, Siegel BA, Dehdashti F, Grigsby PW. The standardized uptake value for F-18 fluorodeoxyglucose is a sensitive predictive biomarker for cervical cancer treatment response and survival. Cancer 2007;110:1738–44.
- Allal AS, Dulguerov P, Allaoua M, Haenggeli CA, El-Ghazi eA, Lehmann W, . Standardized uptake value of 2-[(18)F] fluoro-2-deoxy-D-glucose in predicting outcome in head and neck carcinomas treated by radiotherapy with or without chemotherapy. J Clin Oncol 2002;20:1398–404.
- Downey RJ, Akhurst T, Gonen M, Vincent A, Bains MS, Larson S, . Preoperative F-18 fluorodeoxyglucose-positron emission tomography maximal standardized uptake value predicts survival after lung cancer resection. J Clin Oncol 2004;22:3255–60.
- Cerfolio RJ, Bryant AS. Maximum standardized uptake values on positron emission tomography of esophageal cancer predicts stage, tumor biology, and survival. Ann Thorac Surg 2006;82:391–4.
- Sperti C, Pasquali C, Chierichetti F, Ferronato A, Decet G, Pedrazzoli S. 18-Fluorodeoxyglucose positron emission tomography in predicting survival of patients with pancreatic carcinoma. J Gastrointest Surg 2003;7:953–9.
- Oshida M, Uno K, Suzuki M, Nagashima T, Hashimoto H, Yagata H, . Predicting the prognoses of breast carcinoma patients with positron emission tomography using 2-deoxy-2-fluoro[18F]-D-glucose. Cancer 1998;82:2227–34.
- Tingulstad S, Hagen B, Skjeldestad FE, Halvorsen T, Nustad K, Onsrud M. The risk-of-malignancy index to evaluate potential ovarian cancers in local hospitals. Obstet Gynecol 1999;93:448–52.
- Hubner KF, McDonald TW, Niethammer JG, Smith GT, Gould HR, Buonocore E. Assessment of primary and metastatic ovarian cancer by positron emission tomography (PET) using 2-[18F]deoxyglucose (2-[18F]FDG). Gynecol Oncol 1993;51:197–204.
- Cox D. Regression models and life tables. J R Stat Soc B 1972;34:187–202.
- Kaplan E, Meier P. Non-parametric estimation for incomplete observations. J Am Stat Assoc 1958;53:457–81.
- Avril N. GLUT1 expression in tissue and (18)F-FDG uptake. J Nucl Med 2004;45:930–2.
- Griffiths CT. Surgical resection of tumor bulk in the primary treatment of ovarian carcinoma. Natl Cancer Inst Monogr 1975;42:101–4.
- Hoskins WJ, McGuire WP, Brady MF, Homesley HD, Creasman WT, Berman M, . The effect of diameter of largest residual disease on survival after primary cytoreductive surgery in patients with suboptimal residual epithelial ovarian carcinoma. Am J Obstet Gynecol 1994;170:974–9.
- Aletti GD, Dowdy SC, Podratz KC, Cliby WA. Analysis of factors impacting operability in stage IV ovarian cancer: Rationale use of a triage system. Gynecol Oncol 2007;105: 84–9.
- Chi DS, Venkatraman ES, Masson V, Hoskins WJ. The ability of preoperative serum CA-125 to predict optimal primary tumor cytoreduction in stage III epithelial ovarian carcinoma. Gynecol Oncol 2000;77:227–31.
- de Jong D, Eijkemans MJ, Lie FS, Gerestein CG, Kooi GS, Baalbergen A, . Preoperative predictors for residual tumor after surgery in patients with ovarian carcinoma. Oncology 2007;72:293–301.
- Risum S, Hogdall C, Loft A, Berthelsen AK, Hogdall E, Nedergaard L, . Prediction of suboptimal primary cytoreduction in primary ovarian cancer with combined positron emission tomography/computed tomography—a prospective study. Gynecol Oncol 2008;108:265–70.
- Axtell AE, Lee MH, Bristow RE, Dowdy SC, Cliby WA, Raman S, . Multi-institutional reciprocal validation study of computed tomography predictors of suboptimal primary cytoreduction in patients with advanced ovarian cancer. J Clin Oncol 2007;25:384–9.
- Kumar R, Alavi A. PET imaging in gynecologic malignancies. Radiol Clin North Am 2004;42:1155–67.
- Risum S, Hogdall C, Loft A, Berthelsen AK, Hogdall E, Nedergaard L, . The diagnostic value of PET/CT for primary ovarian cancer—a prospective study. Gynecol Oncol 2007;105:145–9.
- Avril N, Sassen S, Schmalfeldt B, Naehrig J, Rutke S, Weber WA, . Prediction of response to neoadjuvant chemotherapy by sequential F-18-fluorodeoxyglucose positron emission tomography in patients with advanced-stage ovarian cancer. J Clin Oncol 2005;23:7445–53.
- Schwarz JK, Grigsby PW, Dehdashti F, Delbeke D. The role of 18F-FDG PET in assessing therapy response in cancer of the cervix and ovaries. J Nucl Med 2009;50:64–73.
- Bristow RE, del Carmen MG, Pannu HK, Cohade C, Zahurak ML, Fishman EK, . Clinically occult recurrent ovarian cancer: Patient selection for secondary cytoreductive surgery using combined PET/CT. Gynecol Oncol 2003; 90:519–28.
- Risum S, Hogdall C, Markova E, Berthelsen AK, Loft A, Jensen F, . Influence of 2-(18F) fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography on recurrent ovarian cancer diagnosis and on selection of patients for secondary cytoreductive surgery. Int J Gynecol Cancer 2009;19:600–4.