Abstract
Background. Aggressive fibromatosis (AF) is a locally invasive proliferative disease. The mainstay of treatment is surgery. Chemotherapy may be considered in inoperable AF following failure of hormonal therapy and/or NSAIDs. Material and methods. We conducted a retrospective search of the prospectively maintained Royal Marsden Hospital Sarcoma Unit database to identify patients with AF treated with chemotherapy between 1987 and 2009. Results. Thirty-nine patients, thirty one females and eight males, received one or more lines of chemotherapy. The most frequently employed chemotherapy regimens were methotrexate/vinblastine [MTX/VBL] (18) and pegylated liposomal doxorubicin [PLD] (14). MTX/VBL was administered weekly or every two weeks at MTX 50 mg and VBL 10 mg. Treatment duration ranged from three weeks to one year with a median of 4.5 months. Partial response (PR) was observed in 11% of cases, disease stabilisation (SD) in 60% and progressive disease (PD) in 22%. Time to progression ranged from one month to sixteen years. The main toxicities reported were mucositis (4), peripheral neuropathy (3), vomiting (3), and neutropenia (3). PLD was administered at 40–50 mg/m2 every four weeks, for up to six cycles. PR was achieved in 33% and in the remainder the disease was stable with no progression during treatment. Three (25%) patients have so far progressed after treatment. Symptomatic benefit, especially pain relief, was reported in 86% (12/14) of cases. Main toxicities included palmar plantar erythema (5) and mucositis (4). Discussion. MTX/VBL remains a useful combination but PLD is emerging as a well tolerated and effective systemic therapy in advanced AF.
Aggressive fibromatosis (AF), also known as desmoid tumor, is a monoclonal fibroblastic proliferative disease [Citation1] constituting 0.003% of all neoplasms [Citation2]. Despite the absence of metastatic potential, AF may cause debilitating symptoms, deformity and in some cases life threatening organ damage because of its locally invasive behavior. It may occur sporadically or in association with familial adenomatous polyposis (FAP) an autosomal dominant condition characterized by multiple colonic polyps. In sporadic AF, mutations in the beta-catenin gene CTNNB1 lead to increased nuclear expression of beta-catenin, a characteristic feature of the disease [Citation3,Citation4]. FAP – driven AF, on the other hand, is associated with mutations in the APC gene resulting in its inactivation. While FAP-associated AF occurs primarily intra-abdominally, sporadic AF arises predominantly from the extremities.
Asymptomatic patients with static or relatively slowly progressing tumors may remain under observation. Surgery is the treatment of choice [Citation5,Citation6] for symptomatic localized disease, but can often lead to disfiguring results. Recurrence rates following resection can be as high as 30–40% [Citation7]. Radiotherapy may be used as solitary treatment when the disease is deemed inoperable, as consolidation treatment following surgery and as palliative treatment for symptom control [Citation8,Citation9]. When these two modalities are not applicable or fail to control the disease, systemic therapy may be considered [Citation10]. Hormonal agents (anti-oestrogens, aromatase inhibitors) and/or nonsteroidal anti-inflammatory drugs (NSAIDs) have demonstrated modest activity with response rates ranging between 15–30% [Citation11,Citation12]. Other potentially active agents include interferon and more recently, imatinib, a small molecule tyrosine kinase inhibitor [Citation13]. Several combination chemotherapy regimens have demonstrated efficacy but also significant toxicity.
Doxorubicin-based regimens have been commonly used with the combination of doxorubicin with dacarbazine (DTIC) being the most widely reported. The efficacy of this combination has been reported in a number of retrospective studies [Citation11] and more recently in a prospective study [Citation14]. However, serious toxicity associated with the doxorubicin-DTIC regimen, mainly myelosuppression and cardiotoxicity, has been documented [Citation15,Citation16].
The combination of methotrexate (MTX) with vinblastine (VBL) administered on a weekly schedule was first reported to be effective in 1989 [Citation17] and this activity was subsequently confirmed by other investigators [Citation18,Citation19]. Major toxicities with weekly administration included neuropathy and myelosuppression such that the dose interval generally needed to be increased [Citation19]. MTX has also been combined with vinorelbine to reduce the incidence of neurotoxicity [Citation20]. Response rates have been satisfactory not only with these combinations but also with other multi drug regimens including cyclophosphamide and doxorubicin; mitomycin, doxorubicin, and cisplatin; and ifosfamide and etoposide [Citation21].
More recently pegylated liposomal doxorubicin (PLD) has emerged as a therapeutic option in the management of inoperable advanced AF. Evidence to support the activity of PLD in AF was first presented by Wehl et al. in 2004 [Citation22]. In a recent retrospective study performed by our group [Citation23] PLD was shown to be effective and well tolerated.
The aim of the current study was to assess the efficacy and toxicity of systemic chemotherapy in a series of patients with advanced AF treated at a single referral centre. We present a detailed analysis of the subgroups treated with the two most commonly employed regimens, MTX/VBL and single agent PLD – with updated progression data for patients treated with PLD [Citation23].
Material and methods
Patients with progressive or recurrent AF who received one or more lines of chemotherapy between October 1987 and November 2009, were identified from our prospectively maintained database. The majority of patients (over 60%) were referred to our institution following diagnosis, and in certain cases initial management, in other non-specialist centers. Disease status was assessed by clinical examination and imaging (computed tomography or magnetic resonance imaging). Radiological response was assessed using the World Health Organization (WHO) criteria or the Response Evaluation Criteria In Solid Tumors (RECIST) [Citation24]. Changes in disease related symptoms (i.e. pain, limitation of movement) were documented in the majority of cases. Side effects were graded using the National Cancer Institute Common Toxicity Criteria and managed according to departmental protocols for chemotherapy related toxicity. Following completion of treatment the majority of patients remained under regular follow-up comprising clinical/symptomatic and/or radiological assessment of their progress.
Results were analyzed using descriptive statistics. Median and range were used for continuous variables and proportions (%) for categorical variables. The study was reviewed and approved by the Royal Marsden Hospital Clinical Audit Committee.
Results
Thirty-nine patients with symptomatic progressive or recurrent AF received one or more lines of chemotherapy. The female:male ratio was 31:8 and median age at presentation was 27 years (range 3–54). The primary sites of disease included: abdomen/pelvis (11), head and neck (7), limbs (7), chest wall (4), shoulder (4), perineum (2), paraspinal area (2), supraclavicular fossa (1), and axilla (1).
The majority of patients had been previously treated with surgery (30/39 = 77%) and over half of them received radiotherapy (21/39 = 54%) as part of their management. Over 74% (29/39) received hormonal treatment, usually prior to chemotherapy. The most frequently employed chemotherapy regimens were MTX/VBL (18) and PLD (14). Other regimens included doxorubicin/DTIC (5), MTX/other vinca alkaloid (3), ifosfamide (3), vincristine/actinomycin D (2), and other (5).
The details of all previous treatments for the patients who received MTX/VBL and PLD are summarized in and respectively.
Table I. Previous treatments for patients receiving MTX/VBL.
Table II. Previous treatments for patients receiving PLD.
MTX/VBL
Eighteen patients were treated with this combination from May 1992 to December 2005. The majority of patients (16/18, 88%) were chemotherapy naive. Treatment was administered weekly or every two weeks and the starting dose was MTX 50 mg and VBL 10 mg. Treatment duration ranged from three weeks to one year with a median of 4.5 months. PR was observed in 11% (2/18) of cases, stable disease (SD) in 60% (11/18) and progressive disease (PD) in 22% (4/18). Response data were not available for one (6%) patient who was treated elsewhere. Time to progression (TTP) calculated from the start of treatment ranged from one month to sixteen years. Of the patients who achieved PR one had TTP of sixteen years and the other one had TTP of sixteen months. The median TTP for the patients who achieved SD was nine months (range: one month – nine years). Symptomatic benefit was reported in 50% of patients. Toxicity data were incomplete or unavailable (treatment administered elsewhere) in five patients. The main toxicities reported for the remaining patients were mucositis (4), peripheral neuropathy (3), vomiting (3), and neutropenia (3). Toxicity resulted in dose reduction in three cases (in two cases VBL only, in one case both MTX and VBL) and discontinuation of VBL in one case.
PLD
Fourteen patients were treated with PLD between February 2006 and November 2009. PLD was the first chemotherapy regimen employed in 86% (12/14) of patients. It was administered at 40–50 mg/m2 every four weeks, for up to six cycles. The median number of cycles administered was six. Objective response (PR) according to RECIST was achieved in four patients (33%) but notably in some cases not until six to twelve months after completion of chemotherapy [ and ]. In eight cases the disease was stable with no progression during treatment. Two patients were undergoing chemotherapy at the time of the present analysis. Three (25%) patients have so far progressed after treatment and their TTP ranged from nine to twenty months. One of these patients went on to have treatment in the context of a Phase I trial, the second one received single agent hydroxycarbamide and the third one received palliative radiotherapy. The remaining nine patients had no evidence of progression at the end of the follow-up period for this analysis and the median TTP for this group was 27 months (11–45). Symptomatic benefit, especially pain relief, was reported in 86% (12/14) of cases. Main toxicities included palmar plantar erythema (PPE) grade 2 and 3 (5) and mucositis grade 2 and 3 (4). In seven cases (50%) toxicity resulted in dose reduction (in 6 cases from 50 mg/m2 to 40 mg/m2, in 1 case from 40 mg/m2 to 35 mg/m2). A summary of the results is presented in .
Figure 1. Fibromatosis of the right inguinal region. PLD 40 mg/m2 × 6 February 2006 to July 2006. Tumour shrinkage and symptomatic relief. Images: May 2006, November 2006, August 2008.
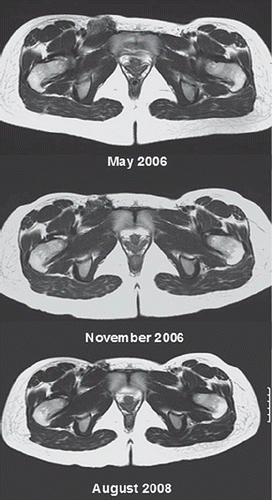
Figure 2. Fibromatosis of brachial plexus PLD 50 mg/m2 × 6 – August 2007 to January 2008, late partial remission, markedly improved mobility of hand and shoulder, with reduced analgesic requirement. Images: August 2007, September 2008, March 2009.
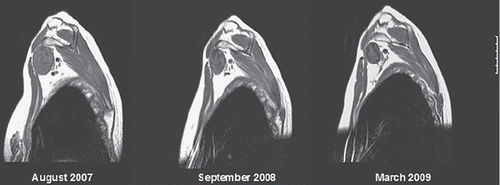
Table III. Treatment with PLD.
Other regimens
Four patients received DTIC/doxorubicin. One patient received the same combination twice. The median number of cycles administered was three. The best response achieved was PR in two cases, SD in two cases and PD in one case. Treatment was discontinued in one case at cycle 5 due to doxorubicin-related cardiotoxicity. One patient had grade 3 neutropenia resulting in dose reduction.
Three patients received single agent ifosfamide; of those one had PD after two cycles, the other two achieved SD (one after 5 and one after 6 cycles). No severe toxicity was reported. Two patients received MTX in combination with vincristine but response and toxicity data were limited as in both cases chemotherapy was administered elsewhere. One patient received MTX/vinorelbine weekly for eight months and achieved SD with nausea/vomiting grade 2 being the main toxicity.
Discussion
A wide variety of chemotherapy regimens have been used in the management of AF, based on evidence from small, predominately retrospective studies. Direct comparison of these regimens in randomized trials is difficult due to the rarity of this disease. Given the lack of metastatic potential, many clinicians remain skeptical about the role of chemotherapy in AF, particularly as chemotherapy may have severe and irreversible complications (including neurotoxicity and cardiotoxicity) and an increased risk of treatment-induced malignancy. Response assessment is another challenging issue in AF treated with chemotherapy especially in static or slow growing tumors as disease stability may represent either benefit from chemotherapy or simply slow progression of the tumor.
In this retrospective study we report our experience with MTX/VBL and PLD, the two most commonly employed chemotherapy regimens in our institution over the last 22 years. In both groups the majority of patients were chemotherapy naïve and MTX/VBL and PLD were the first chemotherapy regimens to be employed in each group. None of the patients had spontaneous regression before treatment. All patients had symptomatic and progressive disease documented either on imaging or on clinical grounds before embarking on chemotherapy.
The MXT/VBL combination demonstrated activity against AF with over half of the patients achieving disease stabilization; however, response duration varied significantly. Only half of the patients reported improvement of symptoms with treatment. Notably a quarter of the patients had disease progression. The fact that other studies [Citation17,Citation18] have reported higher response rates, higher symptomatic response rates and longer median duration of response with MTX/VBL is noted. In the study by Weiss et al. all eight patients had symptomatic relief; two achieved CR (one of which lasted for 30 months), four achieved PR and none of the remaining had PD during treatment. Similarly in the study by Azzarelli et al., of 30 patients treated with MTX/VBL, 40% achieved PR and 60% achieved SD. The ten year progression-free interval was 67%. The difference between our results and the two studies discussed above may be due to the small patient numbers and the heterogeneity of patients included. Furthermore, some of the patients included in our study received treatment at other institutions and consequently, it was not possible to obtain detailed data regarding symptomatic response.
In contrast to the series of patients treated with MTX/VBL, none of the patients treated with PLD progressed whilst on treatment. Objective response was documented in one third of cases and in the remainder the disease was stable by RECIST. Importantly, the vast majority of patients reported clinical benefit in terms of pain control, improved mobility and/or cosmetic improvement. Some patients had late responses with PLD despite the fact that chemotherapy was stopped after six cycles. This observation suggests that continuation of treatment with PLD beyond six cycles, in advanced AF, is not indicated. Response duration was also noticeable. Over a follow-up period of 45 months the median TTP for patients who achieved disease response or stabilization was 27 months.
With regards to toxicity, the combination of weekly/two weekly MTX/VBL was associated with mucositis, neuropathy and neutropenia. In agreement with other studies [Citation18] we observed that in some cases toxicity limited continuation of this regimen. Conversely, most patients treated with PLD tolerated six cycles well. A dose reduction from 50 to 40 mg/m2 was required in nearly half of patients, suggesting that the optimal dose lies between 40 and 50 mg/m2. Mucositis and PPE were the most severe side-effects but were successfully managed with appropriate therapy and dose reduction.
The retrospective nature of this study and the small number of patients involved constitute its main limitations. The two groups are heterogeneous in terms of patient characteristics, primary sites and previous treatments. Over the time frame of this study, response in the group of patients treated with MTX/VBL was assessed using WHO or RECIST criteria, whereas response in all patients treated with PLD was assessed using RECIST. Evaluation according to WHO/RECIST is often difficult owing to the infiltrative nature of the disease and its complex association with adjacent anatomical structures. Alterations in contrast enhancement, indicative of a favorable response may herald symptomatic benefit, although this can be considerably delayed.
Whilst emphasis is increasingly placed on the role of small molecules with more favorable toxicity profile, such as Imatinib13, this retrospective study of a series of unselected patients treated at a single centre, confirms that chemotherapy has a role in the management of patients with unresectable, progressive AF. Direct comparison of patients treated with MTX/VBL and PLD was not possible because of the retrospective nature of the study and the small patient numbers. However, our exploratory results indicate that PLD has comparable activity to other commonly used regimens (particularly MTX/VBL) and is also well tolerated. A randomized trial is required to define the optimal chemotherapy regimen for AF.
Acknowledgements
We are grateful to Cerys Propert-Lewis and Alison Dunlop (clinical nurse specialists), Elizabeth Barquin and Julie Dados (research nurses) for their contribution. There are no conflict of interests from any of the authors.
References
- Alman BA, Pajerski ME, Diaz-Cano S, Corboy K, Wolfe HJ. Aggressive fibromatosis (desmoid tumor) is a monoclonal disorder. Diagn Mol Pathol 1997;6:98–101.
- Bauernhofer T, Stöger H, Schmid M, Smola M, Gürtl-Lackner B, Höfler G, . Sequential treatment of recurrent mesenteric desmoid tumor. Cancer 1996;77:1061–5.
- Tejpar S, Nollet F, Li C, . Predominance of beta-catenin mutations and beta-catenin dysregulation in sporadic aggressive fibromatosis (desmoid tumor). Oncogene 1999;18: 6615–20.
- Alman BA, Li C, Pajerski ME, Diaz-Cano S, Wolfe HJ. Increased beta-catenin protein and somatic APC mutations in sporadic aggressive fibromatoses (desmoid tumors). Am J Pathol 1997;151:329–34.
- Gronchi A, Casali PG, Mariani L, Lo Vullo S, Colecchia M, Lozza L, . Quality of surgery and outcome in extra-abdominal aggressive fibromatosis: A series of patients surgically treated at a single institution. J Clin Oncol 2003;21:1390–7.
- Lev D, Kotilingam D, Wei C, Ballo MT, Zagars GK, Pisters PW, . Optimizing treatment of desmoids tumors. J Clin Oncol 2007;25:1785–91.
- Ballo MT, Zagars GK, Pollack A, Pisters PW, Pollack RA. Desmoid tumor: Prognostic factors and outcome after surgery, radiation therapy, or combined surgery and radiation therapy. J Clin Oncol 1999;17:158–67.
- Nuyttens JJ, Rust PF, Thomas CR, Turrisi AT, 3rd. Surgery versus radiation therapy for patients with aggressive fibromatosis or desmoids tumors. A comparative review of 22 articles. Cancer 2000;88:1517–23.
- Sherman NE, Romsdahl M, Evans H, Zagars G, Oswald MJ. Desmoid tumors: A 20-year radiotherapy experience. Int J Radiat Oncol Biol Phys 1990;19:37–40.
- Janinis J, Patriki M, Vini L, Aravantinos G, Whelan JS. The pharmacological treatment of aggressive fibromatosis: A systematic review. Ann Oncol 2003;14:181–90.
- Patel SR, Benjamin RS. Desmoid tumors respond to chemotherapy: Defying the dogma in oncology. J Clin Oncol 2006;24:11–2.
- Hansmann A, Adolph C, Vogel T, Unger A, Moeslein G. High-dose tamoxifen and sulindac as first-line treatment for desmoid tumors. Cancer 2004;100:612–20.
- Dufresne A, Penel N, Salas S, . Updated outcome with long-term follow-up of imatinib for the treatment of progressive or recurrent aggressive fibromatosis (desmoids tumor): A FNCLCC/French Sarcoma Group phase II trial [abstract 10518]. J Clin Oncol 2009;27:15s.
- Gega M, Yanagi H, Yoshikawa R, Noda M, Ikeuchi H, Tsukamoto K, . Successful chemotherapeutic modality of doxorubicin plus dacarbazine for the treatment of desmoids tumors in association with familial adenomatous polyposis. J Clin Oncol 2006;24:102–5.
- Hartley JA. Alkylating agents. Souhami R, Tannock I, Hohenberger P, . Oxford Textbook of Oncology. 2nd. New York, NY: Oxford University Press Inc; 2002.
- Vadhan-Raj S, Broxmeyer HE, Hittelman WN, Papadopoulos NE, Chawla SP, Fenoglio C, . Abrogating chemotherapy-induced myelosuppression by recombinant granulocyte-macrophage colony-stimulating factor in patients with sarcoma: Protection at the progenitor cell level. J Clin Oncol 1992; 10:1266–77.
- Weiss AJ, Lackman RD. Low-dose chemotherapy of desmoids tumors. Cancer 1989;64:1192–4.
- Azzarelli A, Gronchi A, Bertulli R, Tesoro JD, Baratti D, Pennacchioli E, . Low-dose chemotherapy with methotrexate and vinblastine for patients with advanced aggressive fibromatosis. Cancer 2001;92:1259–64.
- Skapek SX, Hawk BJ, Hoffer FA, Dahl GV, Granowetter L, Gebhardt MC, . Combination chemotherapy using vinblastine and methotrexate for the treatment of progressive desmoid tumor in children. J Clin Oncol 1998;16:3021–7.
- Pilz T, Pilgrim TB, Bisogno G, Knietig R, Koscielniak E, Carli M, . Chemotherapy in fibromatoses of childhood and adolescence: Results from the Cooperative soft tissue sarcoma study (CWS) and the Italian Cooperative study group (ICG-AIEOP). Klin Padiatr 1999;211:291–5.
- Okuno SH, Edmonson JH. Combination chemotherapy for desmoid tumors. Cancer 2003;97:134–5.
- Wehl G, Rossler J, Otten JE, Boehm N, Uhl M, Kontny U, . Response of progressive fibromatosis to therapy with liposomal doxorubicin. Onkologie 2004;27:526–52.
- Constantinidou A, Jones RL, Scurr M, Al-Muderis O, Judson I. Pegylated liposomal doxorubicin, an effective, well tolerated treatment for refractory aggressive fibromatosis. Eur J Cancer 2009;45:2930–4.
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, . New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47.