Abstract
Background. The aim of the present study was to compare both estrogen (ER) and progesterone receptor (PgR) content in operable breast cancers from Vietnamese and Swedish patients. Material and methods. Primary breast cancer tissues were randomly selected from 249 Vietnamese patients treated in Hanoi, Vietnam between 2002 and 2004 and 1 257 Swedish patients treated in Stockholm, Sweden between 2002 and 2003. Clinical information was available for all patients in the study. The hormone receptor content in tumors from Vietnam was analyzed by immunohistochemistry using an automated slide stainer (Bench MarkXT, Ventana) in combination with anti-ER (SP1 250), and anti-PgR (clone 1E2) rabbit monoclonal antibody. Tumors with ≥ 10% stained nuclei were considered as receptor positive. Tumors from Swedish patients were analyzed with an enzyme immunoassay with a cut-off point of ≥ 0.10 fmol/μg DNA as positive. The hormone receptor frequencies between populations were compared according to clincopathology features. Results. The ER positive rate was higher in premenopausal and lower in postmenopausal Vietnamese patients as compared to Swedish patients with similar menopausal status (71% versus 58%, OR 1.75, p = 0.007; 44% versus 72%, OR 0.32, p < 0.001, respectively). PgR positive tumors were found in 58% and 25% of pre- and postmenopausal Vietnamese patients, respectively. The corresponding figures for Swedish patients were 73% and 66%, respectively. Conclusions. There were significant differences in the frequency of ER/PgR positivity between Vietnam and Swedish breast cancer patients. These differences were independent on menopausal status and age of patients at diagnosis can not be explained by these factors and they can be contributed to knowledge about both basic biology features and prognoses.
Breast cancer is at present the most common cancer in women, with more than one million cases occurring worldwide annually. The highest incidence rate, around 84.6–99.4/100,000 population, is reported for industrialized nations such as USA, Australia, and Western Europe, while an intermediate level is reported in Eastern Europe. Asian countries have the lowest level [Citation1]. In Vietnam, the incidence rate is 17.5/100 000 women, while the incidence level for Vietnamese women living in USA is double [Citation2,Citation3].
Recent studies have shown that the ER and PgR content in breast cancer varies between different ethnicities [Citation4–7].
In an early study, Pegoraro et al. reported that hormone receptor status was not different between premenopausal Asian and Caucasian women. In contrast, postmenopausal Asian women had a lower rate of receptor positive tumors as compared to Caucasians [Citation8]. Moreover, it has been reported that Caucasian, Japanese, and Hawaiian women more frequently had hormone receptor positive tumors than women from Vietnam, China and the Philippines [Citation7].
Knowledge about hormonal receptor status of primary breast cancer is essential in predicting treatment response to postoperative adjuvant systemic endocrine therapy according to the recommendation and guideline for operable breast cancer [Citation9]. In patients with recurrent disease, hormone receptor positivity is a good predictor of response to endocrine therapy. Moreover, these receptors also had a prognostic value [Citation10].
Premenopausal Vietnamese and Chinese patients with receptor positive tumors benefited significantly from adjuvant treatment with combined ovarian ablation and tamoxifen treatment [Citation11]. This study was performed in the National Cancer Institute in Hanoi, Vietnam and in other provincial hospitals throughout the country. As a result of this research, immunohistochemical manual staining is now used as a routine practice at the National Cancer Institute in Hanoi and was subsequently approved by the Vietnam Ministry of Health. The aim of the present study was to compare both estrogen and progesterone receptor content in operable breast cancers from Vietnamese and Swedish patients treated over the same period of time.
Material and methods
Clinical materials
Primary breast tumor tissues of 249 Vietnamese patients were randomly selected from Hanoi, Vietnam and 1 257 Swedish patients had information on hormone receptors in the way which corresponds to measurement for Vietnamese patients with histological proven invasive breast carcinoma stage I, II and III. The patients were operated in the National Cancer Institute in Hanoi, Vietnam between 2002 and 2004 and in the Stockholm-Gotland region, Sweden, between 2002 and 2003. For these patients included in this study, information such as age at diagnosis, clinical stage according to UICC (international union against cancer), menopause status, axillary lymph node status, and histopathological type were available. Vietnamese patients who had at least one menstrual period in the last 12 months and Swedish patients six months preceding the operation were defined as premenopausal. All original breast tumor tissues from Vietnam were sectioned to confirm the diagnosis of invasive carcinoma. Patients with stage IV were excluded since there were relatively few Swedish patients in this stage. In addition, patients with in situ carcinoma were also excluded. Methods used for tissue handling, including fixation in Vietnam, were previously described by Love [Citation11].
Hormone receptor assessment
Formaline fixed paraffin embedded tissues were cut at 4 μm thickness and the sections were stored at 2–8°C before immunohistochemical staining. Positive tissue controls were included with every staining set. Specimens from Vietnam were stained at the Cancer Centre Karolinska, Karolinska Institutet, Stockholm (CCK), using the Ventana HX automatic system BenchMark (Ventana Medical Systems, SA, Illkirch Cedex, France), with the following antibodies anti-ER (SP1 250) rabbit monoclonal antibody, (Ventana Medical Systems, SA); anti-PR rabbit monoclonal antibody (clone 1E2) (Ventana Medical Systems, SA). All procedures, including antigen retrieval and blocking of endogenous peroxidase activity were performed automatically by the Benchmark system. The tissue sections were incubated with primary antibody for 32 min at 42°C. Immunoperoxidase staining was performed using the LSAB system NeuVision, according to the manufacturer's instructions (Ventana), and sections were counterstained with hematoxylin eosin. A scoring system in which the proportion of stained cells in each specimen was set up and recorded as 0, < 1%, 1–10%, >10%. Tumors were considered as receptor positive when >10% of the nuclei were stained, irrespective of the intensity as described in European guidelines [Citation9,Citation12]. In cases with invasive carcinoma and carcinoma in situ, the hormone receptors were scored only in the invasive part. Enzyme immunoassay (EIA) utilizing the EIA monoclonal kits (ER-EIA and PgR-EIA, Abbott Laboratories, Abbot Park, IL, USA) was used for assessing hormone receptor in Swedish samples [Citation13]. Tumors with a receptor content ≥ 0.10 fmol/μg DNA were classified in this study as ER and PgR positive. On a subset of the Swedish tumors, receptor analysis was also performed with immunohistochemistry using the SP1 250 anti-ER and the 1E2 anti-PgR monoclonal antibodies. This immunohistochemistry was evaluated by several pathologists as a part of clinical routine. We therefore chose to use the EIA-values which were obtained in a more standardized way.
Statistical analysis
Odds ratios (OR) and 95% confidence intervals were estimated using the logistic regression model to analyze positive hormone receptors in breast cancer tumors for Vietnamese and Swedish patients. Analyses were adjusted for menopausal status alternatively age at diagnosis coded in four categories: ≤40, 41–50, 51–60 and ≥61 and clinical stage.
Person's χ2 was calculated to test differences in frequency distributions for categorical variables. For continuous variables Mann-Whitney U-test was used. All statistical analyses were performed in Stata/IC statistical software version 10.0.
Results
The patient characteristics of both Vietnamese and Swedish women are shown in from which, it can be seen that the Vietnamese patients were significantly younger than Swedish patients (p < 0.001). There were 244 of 249 Vietnamese patients (98%) and 1 174 of 1 257 Swedish patients (93%) with known menopausal status. Sixty four percent of the Vietnames patients were premenopausal while the corresponding figure was 25% for Swedish patients. The mean tumor size as given in the histopathologic reports was 38.7 mm for the cancers from Vietnamese patients compared with 23.7 mm for the Swedish patients. The mean number of removed axillary lymph nodes was 11.0 for the Vietnamese patients whereas, 12% of Swedish patients underwent sentinel lymph node biopsy with maximum three lymph nodes recorded. If we exclude these patients from calculation the mean number excised lymph nodes will be 10.4 (SD = 4.8) for Swedish patients. However, the percentage of patients with metastatic lymph nodes was similar in both groups; 43% for the Vietnamese versus 40% for the Swedish patients. Clinical stage I and II was reported in 10% and 70% respectively of Vietnamese patients. The corresponding figures were 52% and 44%, respectively, for the Swedish patients, p < 0.001 ().
Table I. Patient and tumor characteristic of different study populations.
For the entire study population, the percentage of ER positive tumors was 62% for Vietnamese and 68% for Swedish patients, respectively (). This difference was not statistically significant (p = 0.070). However, when the receptor status was analyzed for pre- and postmenopausal patients it was found that ER positive tumors were seen in 71% of the premenopausal Vietnamese patients as compared to 58% in Swedish patients (). This difference in ER positivity between premenopausal Vietnamese and Swedish patients was statistically significant (p = 0.007). In contrast, 45% of postmenopausal Vietnamese patients had ER positive tumors which was significantly lower than the 72% observed for postmenopausal Swedish patients (p < 0.001). The frequency of ER positivity was calculated for clinical stage I-II-III, and the rates were 71%, 67% and 45% for Swedish patients versus 58%, 62%, and 58% for Vietnamese patients, respectively (data not shown). Thus the rate of ER positive tumors decreased within the advanced stage group in Swedish patients. No such tendency was observed among the Vietnamese patients and no significant differences between the countries were found in ER positive tumors in the different stage, with aspect to age group.
Table II. Comparison of hormone receptor status between Vietnamese and Swedish patients with respect to menopausal status.
We can observe the similar trend for the Swedish patients (the proportions of ER positive tumors in the stage group I, II, and III are: 87%, 76% and 67%). However in this case we have significant differences between the countries in stage group I and II. However the results were chosen to be presented from enzyme immunoassays which were quantitative, and not the results from the evaluation of close gap immunohistochemistry by several different inviduals.
When tumors excised from Vietnam patients were stained also in the National Cancer Institute in Hanoi, 54% were reported as ER positive using a cut-off point of 1%. When the same tumor specimens were stained at CCK, Stockholm, ER-positive tumors were reported as 62% with a cut-off point of 1%. Using a cut-off point of 10%, 62% of the tumors were positive and this cut-off point was chosen for this study as recommended in the European guidelines [Citation12].
From , it can be seen that PgR-positive tumors were observed in 46% of Vietnamese and 68% of Swedish patients. This difference was statistically significant (p < 0.001). When the PgR status was analyzed in pre- and postmenopausal patients, it was found that 58% of Vietnamese premenopausal patients had PR positive tumors, as compared to 73% for Swedish premenopausal patients (p = 0.001). Among postmenopausal patients, PgR positive tumors were observed in 25% versus 66% of Vietnamese and Swedish patients, respectively. Following univariate analysis, the frequency of tumors with the following receptor profiles was compared: ER positive/PgR positive, ER positive/PgR negative, ER negative/PgR positive and ER negative/PgR negative Vietnamese and Swedish patients. The rate of Vietnamese patients with double-positive tumors was lower than for Swedish patients (). In contrast, double-negative tumors were more common among Vietnamese than Swedish patients. Both of these differences were statistically significant.
Table III. Distribution of subtypes by hormone receptor status in the two national populations studied.
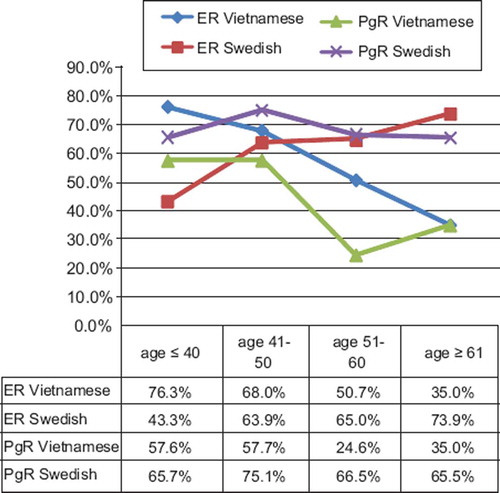
The patients were categorized according to age at diagnosis into four groups: ≤ 40, 41–50, 51–60 and ≥ 61 years of age, and compared with the hormone receptor positivity rates in tumors from Vietnamese and Swedish patients in these different age groups (). From this table we could also see that the odds ratio of having positive ER was more than four times higher for Vietnamese than for Swedish patients < 40 years. This value was decreasing with age and was five times lower for Vietnamese than for Swedish patients in the oldest age group.
Table IV. Odds ratios and 95% confidence intervals for positivity in hormone receptor status between Vietnamese and Swedish patients with respect to age group.
A similar analysis was also performed while controlling for clinical stage with similar results. The PgR status in relation to age of Vietnamese and Swedish patients is shown in . No significant difference at the 5% level was found in patients younger than 40 years old (p = 0.353). But in the other age groups, the odds ratios of PR positive tumors for Swedish patients were significantly higher compared to Vietnamese patients. The trend of ER and PgR positive frequencies according to age group in both populations is revealed clearly in .
Discussion
There have been several reports on the estrogen receptor content in breast cancers tissues for patients of different ethnicities [Citation7,Citation8,Citation14]. The results of the current study have shown that the proportion of receptor-positive tumors in Vietnamese patients tended to be low compared to North European counterparts (Sweden). One study on breast cancers from Thai patients using immunohistochemistry reported 53.4% ER positive and 42.4% PgR positive tumors without specifying the menstrual status of the patients [Citation15]. Similarly, an observation from 186 Malaysian patients showed that 59.1% of tumors were ER positive and 54.8% PgR positive. In this study, 54.8% of the patients were postmenopausal. However, the hormone receptor status for pre- and postmenopausal patients was not reported separately [Citation16]. Vietnamese women living in the Greater San Francisco Bay Area diagnosed with breast cancer tended to be younger than patients of other ethnicities. Furthermore, the Vietnamese patients in question had a higher proportion of ER and PgR positive tumors than Afro-Americans but lower than Caucasian women [Citation3]. To our knowledge, the current study is the first report on both ER and PgR contents in breast cancers from pre- and postmenopausal Vietnam patients. In addition, the results were compared with those from a large group of Swedish breast cancer patients. Our study indicates that ER and PgR positive tumors were less frequent in Vietnamese patients than in Swedish patients, when menstrual status was not taken into account. Previous findings have reported the ER and PgR positive rates of Caucasian living in Northern America were the highest [Citation8,Citation14]. Thus, frequencies of ER positive and/or PgR positive tumors of Vietnamese patients both living in and outside Vietnam were similar to those of other Asian patients and were lower than those previously reported for Caucasian patients.
However, the proportion of receptor-positive tumors appears to be dependent on several factors, of which menstrual status is probably the most important. Hormone receptor status in patients of different ethnicities related to menopausal status has been documented in some previous articles. Hormone receptor positive tumors in postmenopausal Asian patients were less frequent than in their postmenopausal Caucasian counterparts [Citation8]. Thus, Chow et al. reported ER positive tumors were significantly more frequent in postmenopausal than premenopausal Chinese patients [Citation17]. In our current study, most Vietnamese patients (64%) were premenopausal as compared to 25% of the Swedish patients. We found that the frequency of ER positive tumors was 45% for postmenopausal and 71% for premenopausal Vietnamese patients. In contrast, the frequency of ER positive breast cancers was significantly higher than in postmenopausal than premenopausal Swedish patients. In a previous study by Love et al., it was reported that ER positive tumors were found in 62% of premenopausal Vietnamese and Chinese patients which is lower than found in our study [Citation11].
Our results showed that ER positive rates decreased gradually for each 10-year group of Vietnamese patients, from 76 to 35%. Significantly, an opposite trend was observed for Swedish patients. The ER positivity was lowest among patients 40 years old or younger and highest in patients over 61 years, increasing from 43 to 74%. These findings for Swedish patients are consistent to the results of study of over 4 000 breast cancers in Sweden reported by Ferno et al. who found that the rate of ER positive tumors was higher in patients older than 50 years of age than in other age groups sampled [Citation18]. There are only a few previous studies comparing receptor positivity in breast cancers of patients of different ethnicities. One study has shown that the ER positive rates of breast cancer were not statistically significant different between Vietnamese and Australian patients when divided in five-year age groups, except in the group of patients between 51 and 55 years of age [Citation19]. Likewise, no difference in hormone receptor frequency was found when Korean and American patients younger than 45 years old were compared [Citation20]. However, these studies included a limited number of patients with an associated lack of clinical information which makes direct comparison with our findings difficult.
The frequencies of PgR positive tumors from both Vietnamese and Swedish patients were also found to be higher for premenopausal than postmenopausal patients. For Swedish patients, the difference between the two groups was only 7% whereas in premenopausal Vietnamese patients, PgR positive tumors were twice as frequent compared to postmenopausal patients. Moreover, the PgR positive rates were much lower both for pre- and postmenopausal Vietnamese as compared to Swedish patients (p < 0.001). These findings are in agreement with those reported by Joslyn and West who showed that the PgR positive rate in Asian patients was lower than for their Western counterparts [Citation21]. No significant difference in PgR positivity was found between Vietnamese and Swedish patients who were ≤ 40 years. However for patients > 40 years, the PgR positivity of Vietnamese patients was much lower than that recorded for Swedish patients. The maximal difference was observed among patients over 60 years; of these, 35% of the Vietnamese patients had PgR positive tumors as compared to 66% for the Swedish patients. Thus our findings indicated that the rates of PgR positive tumors in Vietnamese patients were significantly lower than for both pre- and postmenopausal Swedish patients. These results are similar to studies on Vietnamese, Thai and Chinese patients [Citation11,Citation14,Citation15,Citation17], when we compared PgR positive rate by stage, no significant difference of PgR positivity was found, except in the case of stage II (i.e. 66% in Swedish versus 46% in Vietnamese patients).
The difference in positive hormone receptor incidence between pre- and postmenopausal Vietnamese patients in our study can not be explained by technical factors such as fixation type and choice of antibody [Citation22,Citation23]. All tumors observed were obtained from patients at one hospital and thus handled postoperatively in a reasonably standardized way. In addition, the receptor staining was carried out in an automated slide staining machine using an antibody which had been tested previously [Citation24] for optimal performance. Assessment of stained slides was done independently by two investigators which reduced the possibility of interpretation errors. It should however be pointed out that the number of postmenopausal Vietnamese patients was limited (85 cases) and the results obtained in the current study for this group need therefore to be confirmed. The receptor data presented for the Swedish patients in the current study were based on enzyme immunoassay. This technique allows a highly standardized quantitative receptor analysis which is not subject to interpretation errors. However, it does not allow for a strict morphological control of the tissue actually analyzed and it is therefore a possible that non-tumorous tissue may in some cases have affected the receptor values obtained. The results using this technique have, however, been compared to that of immunohistochemistry and the results were in good agreement with a concordance of 88% (kappa 0.66) which is in agreement with results published by others [Citation13,Citation25,Citation26]. The observed differences are therefore most likely explained by differences in biological features between tumors from Vietnamese and Swedish patients.
Conclusions
ER positive tumors were more frequent among premenopausal than among postmenopausal Vietnamese patients, as compared to breast cancers from Swedish patients. Furthermore, the ER positivity of Vietnamese patients decreased gradually with rising patient age, which was in contrast to the trend observed for Swedish patients who showed a gradual increase. PgR positivity for Vietnamese was lower than for Swedish patients regardless of age at diagnosis and menopause status. Our findings suggest that a high percentage of young patients in Vietnam could therefore benefit from endocrine therapy. By contrast, postmenopausal Vietnamese patients less often were found to have receptor positive tumors and so the benefit of endocrine therapy in this group is expected to be of limited value.
Acknowledgements
This study was supported by a grant from Sida/SAREC within the Karolinska International Research and Training and the Swedish Cancer Foundation. Access to clinical information and hormone receptor values for the Swedish patients was generously granted by the Stockholm Breast Cancer Study Group. We thank Torsten Hägerström for providing excellent technical assistance and Sinclair H. Mantell for efficiently revising and correcting the English manuscript. This study was approved by the ethical committee of the Karolinska Institute and the Hanoi Medical University, respectively. The authors report no conflict of interest. The authors are responsible for the content and writing of the paper.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74–108.
- Le GM, Gomez SL, Clarke CA, Glaser SL, West DW. Cancer incidence patterns among Vietnamese in the United States and Ha Noi, Vietnam. Int J Cancer 2002;102:412–7.
- Lin SS, Phan JC, Lin AY. Breast cancer characteristics of Vietnamese women in the Greater San Francisco Bay Area. West J Med 2002;176:87–91.
- Gapstur SM, Dupuis J, Gann P, Collila S, Winchester DP. Hormone receptor status of breast tumors in black, Hispanic, and non-Hispanic white women. An analysis of 13,239 cases. Cancer 1996;77:1465–71.
- Althuis MD, Fergenbaum JH, Garcia-Closas M, Brinton LA, Madigan MP, Sherman ME. Etiology of hormone receptor-defined breast cancer: A systematic review of the literature. Cancer Epidemiol Biomarkers Prev 2004;13:1558–68.
- Gown AM. Current issues in ER and HER2 testing by IHC in breast cancer. Mod Pathol 2008;21(Suppl 2):S8–15.
- Miller BA, Hankey BF, Thomas TL. Impact of sociodemographic factors, hormone receptor status, and tumor grade on ethnic differences in tumor stage and size for breast cancer in US women. Am J Epidemiol 2002;155:534–45.
- Pegoraro RJ, Karnan V, Nirmul D, Joubert SM. Estrogen and progesterone receptors in breast cancer among women of different racial groups. Cancer Res 1986;46(4 Pt 2):2117–20.
- Goldhirsch A, Glick JH, Gelber RD, Coates AS, Thurlimann B, Senn HJ. Meeting highlights: International expert consensus on the primary therapy of early breast cancer 2005. Ann Oncol 2005;16:1569–83.
- Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S, . American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol 2007;25:5287–312.
- Love RR, Duc NB, Allred DC, Binh NC, Dinh NV, Kha NN, . Oophorectomy and tamoxifen adjuvant therapy in premenopausal Vietnamese and Chinese women with operable breast cancer. J Clin Oncol 2002;20:2559–66.
- Molina R, Barak V, van Dalen A, Duffy MJ, Einarsson R, Gion M, . Tumor markers in breast cancer – European Group on Tumor Markers recommendations. Tumour Biol 2005;26:281–93.
- Lofgren L, Skoog L, von Schoultz E, Tani E, Isaksson E, Fernstad R, . Hormone receptor status in breast cancer – a comparison between surgical specimens and fine needle aspiration biopsies. Cytopathology 2003;14:136–42.
- Li CI, Malone KE, Daling JR. Differences in breast cancer hormone receptor status and histology by race and ethnicity among women 50 years of age and older. Cancer Epidemiol Biomarkers Prev 2002;11:601–7.
- Lertsanguansinchai P, Chottetanaprasith T, Chatamra K, Sampatanukul P, Wannakrairot P, Rojpornpradit P, . Estrogen and progesterone receptors status in Thai female breast cancer patients: An analysis of 399 cases at King Chulalongkorn Memorial Hospital. J Med Assoc Thai 2002;85(Suppl 1):S193–202.
- Leong BD, Chuah JA, Kumar VM, Yip CH. Breast cancer in Sabah, Malaysia: A two year prospective study. Asian Pac J Cancer Prev 2007;8:525–9.
- Chow LW, Ho P. Hormonal receptor determination of 1,052 Chinese breast cancers. J Surg Oncol 2000;75:172–5.
- Ferno M, Borg A, Johansson U, Norgren A, Olsson H, Ryden S, . Estrogen and progesterone receptor analyses in more than 4,000 human breast cancer samples. A study with special reference to age at diagnosis and stability of analyses. Southern Swedish Breast Cancer Study Group. Acta Oncol 1990;29:129–35.
- Tran D, Lawson J. Rates of estrogen receptor-alpha expression are no different in low-risk (Vietnam) and high-risk (Australian) breast cancer. Appl Immunohistochem Mol Morphol 2004;12:139–41.
- Choi DH, Shin DB, Lee MH, Lee DW, Dhandapani D, Carter D, . A comparison of five immunohistochemical biomarkers and HER-2/neu gene amplification by fluorescence in situ hybridization in white and Korean patients with early-onset breast carcinoma. Cancer 2003;98: 1587–95.
- Joslyn SA, West MM. Racial differences in breast carcinoma survival. Cancer 2000;88:114–23.
- Oyama T, Ishikawa Y, Hayashi M, Arihiro K, Horiguchi J. The effects of fixation, processing and evaluation criteria on immunohistochemical detection of hormone receptors in breast cancer. Breast Cancer 2007;14:182–8.
- Allred DC. Problems and solutions in the evaluation of hormone receptors in breast cancer. J Clin Oncol 2008;26: 2433–5.
- Arihiro K, Umemura S, Kurosumi M, Moriya T, Oyama T, Yamashita H, . Comparison of evaluations for hormone receptors in breast carcinoma using two manual and three automated immunohistochemical assays. Am J Clin Pathol 2007;127:356–65.
- Khoshnoud M, Fohlin H, Fornander T, Stål O, Skoog L, Bergh J, . Immunohistochemistry compared to cytosol assay for determination of estrogen receptor and prediction of long term effect of adjuvant tamoxifen. Forthcoming.
- Ferrero-Pous M, Trassard M, Le Doussal V, Hacene K, Tubiana-Hulin M, Spyratos F. Comparison of enzyme immunoassay and immunohistochemical measurements of estrogen and progesterone receptors in breast cancer patients. Appl Immunohistochem Mol Morphol 2001;9:267–75.