Abstract
Introduction. Little research has been conducted on the effect of self-reported rating of symptom severity on quality of life (QoL) among cancer survivors. The aim of the study was to examine the prevalence of symptoms and whether information about self-reported symptom severity adds value to QoL measurements. Material and methods. A questionnaire including the EORTC QLQ-C30 and an empirically derived symptom check-list was completed by 2 486 cancer survivors participating in a rehabilitation program at baseline and at 1, 6 and 12 months’ follow-up. We used multivariate linear regression models to evaluate the association between QoL and the dichotomous variables for perceived symptom severity (high vs. low) and cancer stage (high vs. low), with adjustment for age, gender, education and time since diagnosis. Results. Of the 2 379 participants who reported having one or more symptoms, 1 479 (62%) considered the reported symptom to be severe. This subgroup had significantly poorer QoL at baseline for all sites, ranging from −15.9 to −10.2, compared to those who did not regard their symptom as severe. Significantly lower baseline levels on all functional subscales were seen for all sites in association with high perceived symptom severity (range from −9.9 to −3.0 (physical functioning), from −21.1 to −13.0 (social functioning), from −18.8 to −8.5 (emotional functioning), and from −18.4 to −9.6 (cognitive functioning). The impairment of physical, social, emotional, and cognitive functioning persisted through 12 months for participants with cancer of the breast, lung and those with lymphomas, although not all reached significance. Discussion. Cancer survivors, irrespective of cancer site, experience a high burden of symptoms. Thorough monitoring and assessment of symptoms and careful scrutiny of cancer survivors’ perceptions of how symptoms affect their lives is critical for clinical identification of patients who might benefit from enhanced medical attention and may be an important supplement to QoL measures.
Although many studies have reported lower health-related quality of life (QoL) of cancer patients experiencing a serious ‘symptom burden’, which includes both cancer-related symptoms, such as fatigue, pain, depression, lack of appetite, or sexual problems [Citation1], and the patient's perception that they interfere with life [Citation2,Citation3], several other studies find that the QoL of cancer survivors appears to be unchanged [Citation4–6]. This may be due to psychosocial adaptation [Citation7], post-traumatic growth [Citation8], or changes in life values [Citation9]. Evidence has emerged, however, that symptoms may persist even if QoL improves [Citation10]. This lack of concordance between symptom burden and QoL may indicate that QoL is influenced by more than symptoms alone. However, it may also indicate that measures of QoL are not sensitive enough to provide sufficient information about symptoms [Citation10,Citation11]. While QoL instruments have made important contributions to therapeutic clinical studies [Citation12], disease-specific measurement of symptoms may, in many cases, be more sensitive and clinically useful for obtaining information about the long-term effects of cancer [Citation10,Citation11]. Better understanding is needed of the relations between measures of symptom burden and of QoL in order to understand how they affect each other [Citation13].
Few studies have been conducted on the effect of self-reported symptom severity on QoL. The occurrence of symptoms varies considerably by the type of cancer, the treatment received, age at treatment, time since treatment, genetic factors, and psychological factors that influence functioning and QoL [Citation1,Citation14]. Furthermore, patients with cancer at an advanced stage may experience more symptoms because they may have received more toxic treatment or have higher tumor burden. Even if a symptom is objectively the same, the subjective experience of the symptom may vary greatly. Some patients may adjust to a symptom and the accompanying limitations, while others may find it unbearable and this may affect the overall QoL.
The aim of the longitudinal study reported here, was to examine the prevalence of symptoms in 2 486 Danish cancer survivors who were participating in a cancer rehabilitation program and investigate whether information about self-reported symptom severity adds value to QoL measurements. We hypothesized that self-reported high severity of symptoms, and high cancer stage at time of diagnosis negatively affects the long-term QoL including physical, emotional, cognitive and social functioning.
Material and methods
The FOCARE study (in Danish: Forskning I Cancer Rehabilitering/Research in Cancer Rehabilitation) has been described in detail previously [Citation15]. In brief, between 2002 and 2005, 3 855 cancer survivors participating in a rehabilitation program at a Danish rehabilitation center were invited to participate in the study aiming to evaluate the long-term effects of a rehabilitation program for cancer patients. The present study is a descriptive part of this study presenting secondary analyses. The general criteria for attending the program were completion of primary treatment and having been evaluated as in need of rehabilitation by the referring physician. There were no restrictions with respect to time since diagnosis or type of cancer. The six-day residential rehabilitation program is conducted by a multidisciplinary team and consists of a combination of lectures and patient group work on themes such as treatment of cancer, psychological reactions, spirituality, sexuality, working life, and lifestyle.
All participants were asked to complete a self-administered questionnaire three weeks prior to (baseline) and 1, 6, and 12 months after finishing the program. The four questionnaires were similar in content except for sociodemographic data, which were collected only at baseline. All participants gave informed consent before inclusion in the study. The study design was approved by the Danish Data Protection Agency.
Exclusions
Of the 3 855 participants, 252 (7%) were excluded for miscellaneous or unknown reasons, 182 (5%) participants had died, and 114 (3%) were ill or hospitalized before follow-up. A further 592 (15%) participants were excluded because they had rare cancer types, which would yield insufficient numbers in each subgroup and thereby low power in the study (a priori set limit at 100 participants before exclusions). A total of 229 (6%) eligible participants did not fill out both the baseline and the 12-month follow-up questionnaires, leaving 2 486 eligible participants in the study.
Clinical and socioeconomic information
Using the unique 10-digit Danish personal identification number assigned to all residents of Denmark by the Central Population Registry in Denmark, we obtained cancer-specific data, such as TNM classification and tumor stage, for each participant by linkage to the Danish Cancer Registry. The Registry contains diagnostic information, classified by an extended Danish version of the International Classification of Diseases 7th edition (ICD-7), and the dates of all cases of cancer in Denmark since 1943 and is considered to be almost complete.
For participants with a diagnosis of cancer of the breast, colorectum or lung or lymphoma, linkage to nationwide population-based clinical databases, the Danish Breast Cancer Cooperative Group [Citation16], the Danish Colorectal Cancer Group [Citation17], the Danish Lung Cancer Group [Citation18], and the Danish National Lymphoma Database [Citation19], provided detailed clinical information on date of diagnosis, tumor stage and treatment. For participants with cancer in the prostate, cervix or ovary or head and neck, a project nurse reviewed the medical hospital records and extracted the date of diagnosis, tumor stage, and treatment.
Self-reported information on education, employment status, and marital status was obtained from the baseline questionnaire. Information on symptom burden and severity of reported symptoms were obtained both at baseline and at the 12-month follow-up [Citation15].
Outcome measures
QoL was measured with the Danish version of EORTC QLQ-C30, a validated, widely used questionnaire that covers five functional scales (physical, role, cognitive, emotional, and social), three symptom scales (fatigue, pain, and nausea and vomiting), one global health status/QoL scale, perceived financial impact of the disease, and six single items (including dyspnea, loss of appetite, and insomnia). On the functional scales, a higher score represents a higher level of functioning, with a maximum of 100 points [Citation20]. We used the global QoL (two items) and four functional scales: physical (five items), cognitive (two items), emotional (four items) and social (two items).
Symptom burden was reported on an empirically derived symptom check-list as whether the symptom was present and, if so, whether it was present before the diagnosis of cancer. The symptoms listed were the 18 most frequently reported symptoms associated with cancers in the breast, colon and rectum, cervix and ovary, lungs, head and neck (oral cavity, salivary glands, thyroid gland, larynx, pharynx, and esophagus), prostate and lymphomas; the symptoms were: fatigue, lack of concentration, memory lapses, hot flushes, sensory disturbances in the hand or feet, sleep interruption, dry mucous membranes, digestive problems, swelling of limbs, dyspnea, pain in joints or muscles, impaired mobility, dental problems, weight gain or loss, altered sense of taste or smell, urinary problems, sexual problems, and potency problems. If a participant suffered from one or more of the predefined symptoms, further questions asked for a rating of the severity of the symptom on a four-point scale, ranging from ‘very bothersome’, ‘somewhat bothersome’, ‘a little bothersome’, to ‘not bothersome’.
Stratification of groups
In order to investigate the potential influence of self-reported symptom severity on QoL, we formed two subgroups: the first consisted of participants registered as having any of the predefined symptoms but perceived them to be ‘somewhat’, ‘a little’, or ‘not’ bothersome; the second group were those with any of the symptoms and considered them ‘very’ bothersome. This dichotomization was made because we were interested in identifying the most affected participants and initial analyses revealed that almost all participants had reported at least one of the pre-defined symptoms to be either ‘somewhat ‘or ‘very’ bothersome.
In order to examine the association between advanced stage of cancer and QoL, the participants were stratified into high (regional spread or disseminated disease) and low stage. The variable was based on information from the clinical database or medical charts, depending on the tumor site, and subsequently on information from the Danish Cancer Registry. Participants with breast cancer were classified as high stage if they had tumor-positive lymph nodes or were N1-3 or M1 in the TNM classification. Lymphomas were classified as high stage if the Ann Arbor stage was III or IV, ECOG performance status was 2–4, and B symptoms were present. Prostate cancers were classified as high stage if the Gleason score was > 6; the remaining tumor sites (lung, colorectal, head and neck cancers, and female genital organs) were classified as high stage if they were N1–3 or M1 in the TNM classification.
Statistical analyses
Multivariate linear regression models were used to evaluate the association between the baseline values of EORCT QLQ C30 subscales and tumor stage (high vs. low) and self-reported symptom severity (high vs. low). To estimate the impact of stage of cancer and self-reported symptom severity, the QoL including the functioning scales were compared on differences between scores at baseline and 12 month follow-up. Negative differences (−) on the scales represented poorer scores and positive differences (+) represented improvement in QoL or functioning.
Two models were run, with QoL and functioning scales as dependent variables and stage and symptom severity as independent variables. All models were adjusted for age at baseline, gender, education and time since diagnosis. In the models examining change between baseline and the 12-month follow-up, we also adjusted for the baseline score of the given subscale.
Except for the QoL subscale ratings, data from the functional scales in the baseline questionnaire did not follow a normal distribution. We therefore tested the data with a non-parametric test (Wilcoxon). As the results varied only minimally from those of parametric tests, we decided to use only the parametric tests in this study because it allows for precise estimation of differences.
For the continuous variables (age, baseline score of each scale, and time since diagnosis), the linearity of the association with the outcome was evaluated in a linear spline with knots placed at the deciles [Citation21]. We found no departure from linearity.
Results
Most of participants were women (85%), of whom 73% had had breast cancer. The majority of the participants (53%) had cancer at high stage at baseline; however, considerable intergroup differences were observed, ranging from 88% of participants with head and neck cancer to 24% of those with cancer of the lung (). A total of 65% of the participants were married or cohabitating, ranging from 84% of participants diagnosed with prostate cancer to 55% with head and neck cancers. Most of the participants were working (62%), only 6% being on sick leave or unemployed. Generally, the participants were well educated, and most had youth (38%) or higher (50%) education, according to the International Standard Classification of Education [Citation22]. Most (40%) reported their annual household income as medium (33.550–73.670 Euros) (data not shown).
Table I. Disease-related and demographic characteristics at baseline of 2486 participants in the FOCARE study by cancer site.
Generally, all participants reported high frequencies of the 18 predefined symptoms (). A total of 2 379 (96%) of the participants reported having one or more of the symptoms (ranging from 100% of participants with head-and-neck cancers to 91% of participants with colorectal cancer). Among these, 1 479 (62%) considered the reported symptom to be severe, and the frequencies of perceived high symptom severity for specific sites were high, ranging from 77% of participants with head-and-neck cancers to 49% of those with colorectal cancer. For cancers at all sites fatigue was the most occurring symptom and ranged from 37% (prostate cancer) to 77% (lung cancer). Except for prostate cancer, high frequencies of lack of concentration ranging from 32% (colorectal cancer) to 55% (lung and head and neck cancer), memory lapses (from 35% (colorectal cancer) to 52% (head and neck cancer), sleep interruption (from 32% (colorectal cancer) to 64% (lymphomas), and joint or muscle pain (from 37% (lung cancer) to 91% (colorectal cancer) were reported ().
Table II. Prevalence (percentages) of self-reported cancer-related symptoms and symptom severity among 2 486 participants in the FOCARE study by cancer site.
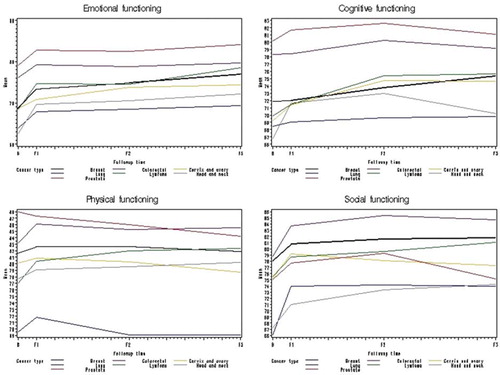
shows a small but stable increase in mean global QoL between baseline and the 1-month (F1) and 6-month (F2) follow-up for almost all cancer sites; however, at the 12-month follow-up (F3), the QoL decreased for participants with cancer of the prostate, lung, cervix or ovary. Participants with lung cancer reported the lowest mean scores at baseline through F1, F2 and F3 with means at 53.7 (SD 20.2), 54.8 (SD 21.9), 54.1 (SD 22.8), and 53.3 (SD 21.7) respectively. Participants with prostate cancer scored the highest with means at 69.8 (SD 21.0), 71.2 (SD 18.4), 72.0 (SD 20.1), and 68.7 (SD 23.2) respectively. shows small changes in the functional scales of the EORTC QOQ-C30 between baseline and the three follow-up times.
Figure 1. Mean scores on EORTC QLQ-C30 quality of life subscale at baseline and at 1-, 6-, and 12-month follow-up by cancer site B: Baseline; F1: 1 month follow-up; F2: 6 month follow-up; F3: 12 month follow-up.
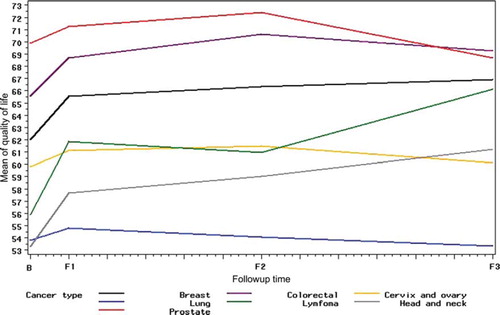
Figure 2. Mean score on EORTC QLQ-C30 functioning subscales at baseline and at 1-, 6-, and 12-month follow-up by cancer site B: Baseline; F1: 1 month follow-up; F2: 6 month follow-up; F3: 12 month follow-up.
In adjusted models of baseline levels and changes in physical, social, emotional, and cognitive functioning at 12 months in participants with high-stage cancer and high self-reported symptom severity (), no clear pattern was seen for any site in relation to stage of cancer. The subgroup of participants who had reported a symptom and regarded it as severe had significantly poorer global QoL at baseline for all sites compared to those who did not regard their symptom as severe ranging from −15.7 (lymphomas) to −10.2 (breast cancer) (); however, at the 12-month follow-up, they reported only slightly poorer QoL, with significant results only for those with cancers of the breast and lung.
Table III. Multivariate linear regression models of the association between tumor stage (high vs. low) and self-reported symptom severity (high vs. low) and baseline values of EORTC QLQ-C30 subscale (model 1) and change between baseline and the 12-month follow-up (model 2) of 2 486 participants in the FOCARE study by cancer site.
Significantly lower baseline levels on all functional subscales were seen for all sites in association with high perceived symptom severity than for participants who did not consider their symptom to be severe (range from −9.9 to −3.0 (physical functioning), from 21.1 to −13.0 (social functioning), from −18.8 to −8.5 (emotional functioning), and from 18.4 to −9.6 (cognitive functioning) (). However, at 12 months the impairment of physical, social, emotional, and cognitive functioning only persisted to be significant for participants with cancer of the breast. Participants with cervix and ovary cancer who considered their symptom as severe reported significantly lower physical- and social functioning at 12 months follow-up than those who did not consider their symptom as severe. Participants with lung cancer reported significantly lower on physical- and emotional functioning if they considered their symptom to be very bothersome. Participants with lymphomas who considered their symptom to be severe scored significantly lower on social functioning at 12 months follow-up. In the other cancer sites no differences were revealed ().
Discussion
In this study of 2 486 cancer survivors, we found high frequencies of symptoms associated with cancers at all sites. Most of the participants who reported that they had at least one symptom, which they perceived to be severe, had significantly lower scores for both QoL and functioning scales at baseline than participants who did not find their symptom very bothersome. A similar pattern was seen at the 12-month follow-up for participants with cancers in the breast, lung and those with lymphomas, although not all reached significance. In the other cancer sites no clear differences between the two groups were revealed. No clear association was found between cancer stage and QoL or functional scales, which may indicate that QoL refers to more than disease-related variables.
These results show that self-perceived symptom severity is important for daily functioning and well-being, and incorporation of more detailed measures of self-reported symptom burden into QoL instruments might therefore provide more precise information about the suffering of cancer patients during or after treatment. Detailed measurement of symptom burden might also nuance the reports of unchanged or enhanced QoL after cancer. A growing number of reports indicate that although symptoms contribute to the construct of QoL, they do not determine it because QoL refers to more than symptoms [Citation3,Citation10,Citation13]. Results obtained with instruments measuring generic QoL might be more influenced by factors that are not directly related to the disease or its treatment (e.g. socioeconomic position) than measures of symptom burden. Measures of symptom burden might provide less information than measures of QoL, but the information is more closely related to the disease and its treatment, thus better reflecting the various stages of survivorship [Citation11,Citation12]. In a review of clinical trials of QoL and symptom management, Buchanan and colleagues questioned the extensive use of QoL measurements in symptom management trials and concluded that its added research value remains to be established [Citation13]. However, in another systematic review to evaluate instruments for symptom assessment, Kirkova et al. found that QoL instruments are often substituted for those for symptom assessment [Citation3], which may yield discrepant results that could compromise outcomes.
Our results are in line with other studies. Kirkova et al. [Citation14] found a strong relationship between symptom severity and symptom distress in a study among 181 advanced cancer patients with multiple cancer symptoms, and that the prevalence of distress increased with greater symptom severity. However, one third of the patients with clinically mild symptoms considered them distressing suggesting that only a comprehensive symptom assessment can capture the total burden experienced by an individual. In a study among 590 Dutch cancer patients in the palliative phase, Hoekstra et al. [Citation23] examined the added value of assessing the “most troublesome” symptom (the symptom which was causing the most trouble in the patients everyday life) in addition to presence and severity of symptoms commonly assessed in clinical practice. The authors found that in 18% of the cases there was no congruence between the clinical assessment of presence and severity of a symptom and the patients’ own experience of the distress and interference of the symptom in their everyday life [Citation23]. Each individual experiences cancer differently. Therefore, measurement of the cancer-specific symptom burden and patients’ rating of the severity of their symptoms might be an important supplement to QoL measures in clinical tracking and evaluation of the short- and long-term effects of cancer.
It is notable that 38% of the cancer patients who reported one or more of 18 symptoms did not evaluate this symptom as very bothersome. This may indicate that patients cope well with the symptom or that patients do not consider the symptom as an important factor in everyday life. In line with this argument one may suggest that this group may be patients in which one or more of the complaints present with fewer or less severe symptoms compared to the remaining 62% of cancer patients.
The findings of this study should be considered in the light of its limitations. First, we had no objective measures of symptoms. Further, all the participants had been evaluated as in need of rehabilitation by their physician, which might indicate that they had particular problems or persistent distress. Thus, the self-reported symptom burden and perceived severity probably do not represent those of the entire population of cancer survivors. The fact that the majority of the participants were well educated with a medium household income also indicates that they represent a selected group. As a consequence, our findings cannot be generalized to all cancer survivors. As within-group analyses were conducted only among participants who reported having one or more symptoms, however, potential selection bias was minimized.
Secondly, we had no information on comorbidity, which may affect the symptom burden in a dose-dependent manner [Citation24]. In a population-based study of 1904 cancer survivors and 29 092 non-cancer controls in the USA, Mao et al. investigated the effect of comorbidity (measured as the number of health conditions that caused functional limitations) and age on self-reported symptom burden (pain, psychological distress, and insomnia). They found that cancer survivors reported a higher symptom burden as a function of increasing number of comorbid conditions than controls and that the overall symptom burden increased significantly with the number of comorbid conditions [Citation24]. It is therefore possible that some of the reported symptoms and symptom severity in our study were due to medical conditions other than cancer. However, symptoms can arise from cancer treatment, the disease itself, other medical conditions, acute injuries, or combinations of all or some of the causes [Citation1,Citation10,Citation11], and it is not always possible to disentangle the causes of a given symptom. Adjustment for medical conditions other than cancer would probably lead to an underestimation of the true effect.
Most of the symptoms reported in our study were probably related to treatment regimes, although some might have been directly associated with the location or type of cancer. Our finding that most of the participants experienced high frequencies of fatigue, sleep interruption, joint or muscle pain, and weight gain or loss are in accordance with the findings of other studies, which have almost all recognized this as a symptom cluster [Citation10,Citation25]. There has been some debate about whether symptom clusters have a common underlying biological mechanisms, e.g. an inflammatory response produced by the disease or its treatment, or whether clustering might be explained as a symptom cascade, in which one symptom is the cause of the other (e.g. pain leads to fatigue which leads to depression), or by patient-related factors, such as medical history or genetic disposition [Citation11,Citation25]. More research is needed on symptom clusters and their potential influence on long-term cancer survivorship.
The strengths of this study include the large sample, the long follow-up time, the extensive use of registry-based data from detailed clinical databases and the national Danish Cancer Registry, and the detailed questionnaire which provided a comprehensive list of cancer-related symptoms, perceived symptom severity, and socioeconomic data. Furthermore, we were able to control for a number of variables that have been found to be associated with QoL, such as age, time since diagnosis, gender, and education.
Conclusions
Cancer survivors, irrespective of the site of cancer, experienced a high burden of symptoms, and self-reported symptom severity at baseline negatively affected QoL including physical, emotional, cognitive and social functioning. This impairment in QoL continued through 12-month follow-up for participants with cancer in the breast, lung and those with lymphomas although not all results reached significance. In the other cancer sites no clear differences were revealed. No clear pattern emerged with regard to stage of cancer.
Health-care professionals should be aware of the considerable symptom burden experienced by patients. Thorough monitoring and assessment of symptoms and careful scrutiny of cancer survivors’ perceptions of how symptoms affect their lives are critical for clinical identification of patients who might benefit from enhanced medical attention and may be an important supplement to generic QoL measures.
Acknowledgments
This study was funded by the Danish Cancer Society. We wish to thank the cancer patients who participated in the study for their valuable contributions. We also wish to acknowledge the important collaboration with the staff at the Dallund Rehabilitation Centre and the participating hospital departments.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Stein KD, Syrjala KL, Andrykowski MA. Physical and psychological long-term and late effects of cancer. Cancer 2008;112(11 Suppl):2577–92.
- Cleeland CS, Reyes-Gibby CC. When is it justified to treat symptoms? Measuring symptom burden. Oncology (Williston Park) 2002;16(9 Suppl 10):64–70.
- Kirkova J, Davis MP, Walsh D, Tiernan E, O'Leary N, LeGrand SB, . Cancer symptom assessment instruments: A systematic review. J Clin Oncol 2006;24:1459–73.
- Hammerlid E, Taft C. Health-related quality of life in long-term head and neck cancer survivors: A comparison with general population norms. Br J Cancer 2001;84:149–56.
- Johansson B, Brandberg Y, Hellbom M, Persson C, Petersson LM, Berglund G, . Health-related quality of life and distress in cancer patients: Results from a large randomised study. Br J Cancer 2008;99:1975–83.
- Mols F, van de Poll-Franse LV, Vingerhoets AJ, Hendrikx A, Aaronson NK, Houterman S, . Long-term quality of life among Dutch prostate cancer survivors: Results of a population-based study. Cancer 2006;107:2186–96.
- Lechner SC, Carver CS, Antoni MH, Weaver KE, Phillips KM. Curvilinear associations between benefit finding and psychosocial adjustment to breast cancer. J Consult Clin Psychol 2006;74:828–40.
- Manne S, Ostroff J, Winkel G, Goldstein L, Fox K, Grana G. Posttraumatic growth after breast cancer: Patient, partner, and couple perspectives. Psychosom Med 2004;66:442–54.
- Bower JE, Meyerowitz BE, Desmond KA, Bernaards CA, Rowland JH, Ganz PA. Perceptions of positive meaning and vulnerability following breast cancer: Predictors and outcomes among long-term breast cancer survivors. Ann Behav Med 2005;29:236–45.
- Burkett VS, Cleeland CS. Symptom burden in cancer survivorship. J Cancer Surviv 2007;1:167–75.
- Cleeland CS. Symptom burden: Multiple symptoms and their impact as patient-reported outcomes. J Natl Cancer Inst Monogr 2007;37:16–21.
- Ferrans CE. Differences in what quality-of-life instruments measure. J Natl Cancer Inst Monogr 2007;37:22–6.
- Buchanan DR, O'Mara AM, Kelaghan JW, Minasian LM. Quality-of-life assessment in the symptom management trials of the National Cancer Institute-supported Community Clinical Oncology Program. J Clin Oncol 2005;23: 591–8.
- Kirkova J, Walsh D, Rybicki L, Davis MP, Aktas A, Tao J, . Symptom severity and distress in advanced cancer. Palliat Med 2010;24:330–9.
- Hoybye MT, Dalton SO, Christensen J, Larsen LR, Kuhn KG, Jensen JN, . Research in Danish cancer rehabilitation: Social characteristics and late effects of cancer among participants in the FOCARE research project. Acta Oncol 2008;47:47–55.
- Moller S, Jensen MB, Ejlertsen B, Bjerre KD, Larsen M, Hansen HB, . The clinical database and the treatment guidelines of the Danish Breast Cancer Cooperative Group (DBCG); its 30-years experience and future promise. Acta Oncol 2008;47:506–24.
- Harling H, Bulow S, Kronborg O, Jorgensen T. Treatment of rectal cancer in Denmark 1994–1999. Ugeskr Laeger 2004;166:368–71. Danish.
- Jakobsen E, Palshof T, Osterlind K, Pilegaard H. Data from a national lung cancer registry contributes to improve outcome and quality of surgery: Danish results. Eur J Cardiothorac Surg 2009;35:348–52.
- Danish Lymphoma Registry. Year Report 2008. Danish Lymphoma Group [Internet]. 2008. Available from: http://www.lymphoma.dk.
- Osoba D, Aaronson N, Zee B, Sprangers M, Te VA. Modification of the EORTC QLQ-C30 (version 2.0) based on content validity and reliability testing in large samples of patients with cancer. The Study Group on Quality of Life of the EORTC and the Symptom Control and Quality of Life Committees of the NCI of Canada Clinical Trials Group. Qual Life Res 1997;6:103–8.
- Greenland S. Dose-response and trend analysis in epidemiology: Alternatives to categorical analysis. Epidemiology 1995; 6:356–65.
- UNESCO [Internet]. Paris: International Standard Classification of Education; 2010. Available from: http://www.unescoorg/en/education.
- Hoekstra J, Vernooij-Dassen MJ, de VR, Bindels PJ. The added value of assessing the ‘most troublesome’ symptom among patients with cancer in the palliative phase. Patient Educ Couns 2007;65:223–9.
- Mao JJ, Armstrong K, Bowman MA, Xie SX, Kadakia R, Farrar JT. Symptom burden among cancer survivors: Impact of age and comorbidity. J Am Board Fam Med 2007;20:434–43.
- Buchanan DR, O'Mara AM, Kelaghan JW, Sgambati M, Caskill-Stevens W, Minasian L. Challenges and recommendations for advancing the state-of-the-science of quality of life assessment in symptom management trials. Cancer 2007;110:1621–8.
