Abstract
We evaluated factors associated with use of antidepressant medication subsequent to a diagnosis of breast cancer. We also evaluated the effect of participation in a cancer rehabilitation program on use of antidepressants. Material and methods. We conducted a register-based cohort study of 1 247 women with breast cancer diagnosed between 1998 and 2006 who attended a week-long rehabilitation program and a comparison group of 2 903 women who did not attend the program matched through the registers of the Danish Breast Cancer Cooperative Group. The associations between breast cancer-related, treatment-related, and sociodemographic factors and use of antidepressants were evaluated in multivariate Cox proportional hazard models separated on use of antidepressants before diagnosis of breast cancer. Results. The mean follow-up for the 4 150 women in the study was 3.3 years (5–95% range, 0.3–7.0 years) and 1 020 (25%) were users of antidepressants after diagnosis of breast cancer. Among women who had not used antidepressants before their breast cancer, the diagnosis of a new primary cancer increased the adjusted hazard ratio (HR) to 3.34 (95% CI, 1.50–7.76), and recurrence of breast cancer increased the HR for first use of antidepressants to 2.56 (95% CI, 1.86–3.52). Unemployment was associated significantly with use of antidepressants, whereas having no children living at home, lower income, and the number of tumor-positive axillary lymph nodes were of borderline significance. No effect of the rehabilitation program was observed on first use of antidepressants after breast cancer. Discussion. Diagnosis of a new cancer or recurrence of breast cancer considerably increased the rate of use of antidepressants. Sociodemographic rather than disease- or treatment-related characteristics at the time of diagnosis were associated with first use of antidepressants after a breast cancer diagnosis.
In view of the increasing incidence of breast cancer throughout the twentieth century and a constantly improving survival rate in industrialized countries [Citation1], it is important to understand the immediate as well as long-term effects of breast cancer, which may affect the psychological, social, and physical aspects of the quality of life of survivors [Citation2].
Numerous investigations have reported a high incidence of depression and anxiety among breast cancer patients [Citation3]. Aside from being a serious disorder in its own right, depression is associated with poor prognosis of physical disease and co-occuring depression in cancer patients may be associated with increased mortality [Citation4] possibly related in part to reduced treatment compliance among depressed cancer patients [Citation5]. In the clinical cancer setting, the challenge is to identify cancer patients in need of treatment for clinical depression and this condition has been reported to be underdiagnosed and undertreated in patients with cancer [Citation6–8]. It would therefore be useful to identify the predictive factors that are consistently linked to depression in these patients, and numerous studies have sought to do so in breast cancer patients [Citation8–14]. Treatment modality and biological characteristics of breast cancer (such as stage at diagnosis) were associated with depression in some [Citation8,Citation9,Citation13] but not all studies [Citation10]. Comorbidity [Citation8,Citation13,Citation14], and severity of physical symptoms , including insomnia [Citation9,Citation10], pain [Citation14], and fatigue [Citation9], have also been associated with depression, whereas age only inconsistently influenced depression in breast cancer patients [Citation8,Citation13,Citation14]. Education and income were also found to influence the risk for depression in such patients [Citation8,Citation9,Citation11]. As in the general population, prior depression is strongly associated with new depressive episodes in patients with breast cancer [Citation8,Citation12]. Few studies had the possibility to involve information on both characteristics of disease, treatment, comorbidity and social factors.
While most studies used psychometric evaluation of depression, two US studies operationalized depression by use of antidepressants among breast cancer patients [Citation13,Citation14]. These studies were however, limited by inclusion of only insured women [Citation13] and small number of patients [Citation14].
Antidepressant medication is predominantly prescribed for depression and secondly for anxiety disorders [Citation15,Citation16]. Use of antidepressant medication was used as a marker for the recognized need for treatment of depression and anxiety disorders in breast cancer patients. In the register-based cohort study reported here, we evaluated if a range of factors related to breast cancer, its treatment, comorbidity and socioeconomic position were associated with use of antidepressants after a diagnosis of breast cancer in more than 4 000 women. Further we investigated the use of antidepressants before and after participation in a rehabilitation program.
Material and methods
Study population
We identified all 1 277 women in whom breast cancer was diagnosed between January 1, 1998, and December 31, 2006, and who attended a six-day rehabilitation course at the Dallund Rehabilitation Centre in the period 2002–2007. Participants at the rehabilitation course had to have completed primary treatment, have a need for rehabilitation, and physical ability to participate in the activities offered. The rehabilitation program has been described in detail by Høybye and colleagues [Citation17]. Briefly, the retreat included patient group work and lectures on themes such as treatment of cancer, psychological reactions, spirituality, working life, and lifestyle. Each day, the participants were involved in a combination of physical activities and lectures.
Through the files of the Danish Breast Cancer Cooperative Group (DBCG) we identified a comparison group of 3 040 women with breast cancer who did not attend the rehabilitation course, individually matched on age, date of diagnosis, and treatment protocol on a 2:1 basis. The DBCG has since 1977, registered almost 95% of all breast cancers diagnosed in Danish women below the age of 75 years and conducted protocol-based randomized trials of surgery, radiation, chemotherapy, and endocrine therapy in patients with primary invasive breast cancer [Citation18].
For the total of 4 317 women in the study, we obtained date of diagnosis (defined as date of surgery), clinical variables, treatment modalities, information on recurrence, and diagnosis of new primary cancer from the DBCG.
We excluded 166 women for whom there was no information on tumor size or lymph node status and one woman who attended the rehabilitation course after a diagnosis of ovarian cancer but before her diagnosis of breast cancer, leaving a final study population of 4 150 women, with 1 247 attending the Dallund Rehabilitation Centre and 2 903 in the comparison group. The sample represented more than 10% of all breast cancers diagnosed in the country during the study period.
Information on sociodemographic factors, comorbidity, and vital status
We obtained individual information on level of attained education, income, affiliation to the work market, partners, and number of children aged below 18 years living at home for each of the years 1996–2007 by using the Integrated Database for Labor Market Research, a research database coordinated by Statistics Denmark. The database contains a variety of demographic and socioeconomic information on the entire Danish population and is updated annually at the end of each year [Citation19]. Information about comorbidity was obtained from the Danish National Health Registry, which, since 1977, has registered information about all patients admitted to hospitals in Denmark and includes both administrative data and information about treatment and diagnosis [Citation20]. We computed the Charlson comorbidity index for each woman from 1978 onwards [Citation21]. The socioeconomic information and the Charlson comorbidity index for the year before the breast cancer were used in the analyses.
From the civil registration system, we obtained information on death, emigration, and disappearance through 2007.
Information on use of antidepressants
The National Prescription Drug Database contains information on prescriptions for drugs dispensed at all pharmacies in Denmark from 1995 onwards. The available information includes the type of drug prescribed according to the Anatomical Therapeutic Chemical (ATC) classification system and date of issue from the pharmacy [Citation22]. In Denmark, antidepressants are available only on prescription; we obtained information on all prescriptions for antidepressants in the ATC group N06A from 1995 through 2007. Use of antidepressants was defined as two or more independently redeemed prescriptions.
Statistical analyses
The χ2 test, Student's t-test, and the log rank test were used to evaluate differences in baseline characteristics between the group of women who attended the Dallund Rehabilitation Centre and the matched comparison group.
Cox proportional hazard models were used to analyze factors associated with the use of antidepressants after a diagnosis of breast cancer, with time since breast cancer diagnosis as the underlying time scale. Age was stratified at < 40, 40–45, 45–50, 50–52.5, 52.5–55, 55–60, and > 60 years. Women were followed from date of diagnosis of breast cancer until death, emigration or December 31, 2007 whichever came first. As preliminary analyses confirmed that use of antidepressants before a diagnosis of breast cancer was strongly associated with use of these medications after diagnosis, we separately analyzed the group of women who had used antidepressants between January 1, 1995, and one year before the breast cancer diagnosis and those who had not.
The covariates included were clinical variables (tumor size, tumor-positive axillary lymph nodes, menopausal status, estrogen receptor status, and grade of malignancy); treatment-related factors (type of surgery and adjuvant treatment: chemotherapy, antihormonal therapy, and radiation); sociodemographic factors (age, level of education, income, work market affiliation, cohabitation status, number of children living at home); and the Charlson comorbidity index. Recurrence of breast cancer and diagnosis of a new primary cancer after breast cancer were entered as time-dependent variables.
The proportional hazard assumption was evaluated for each categorical covariate graphically by a plot of log minus log of the survival density function versus log of follow-up time. After visual assessment of whether the curves were approximately parallel, no variable was considered to violate the proportional hazard assumption. Continuous variables (tumor size, number of positive lymph nodes, and income) were tested with the proportionality test feature of PROC PHREG in SAS version 9.1. The linearity of associations was evaluated graphically by linear splines with the boundaries placed at the percentiles 10–90 in 10% steps; all continuous variables were entered linearly into the model. We created models mutually adjusted for all covariates. As the comparison group was expected to represent the general population of breast cancer patients, we ran the Cox models both for the comparison group separately as well as for the full sample.
Even if the covariates included in our analysis were similarly associated with use of antidepressants by the women who attended the rehabilitation program and the comparison group and we had decided to stratify all analyses according to previous use of antidepressants, we were still concerned that there might be an overall difference in use of antidepressants at a group level. We plotted unadjusted and adjusted Kaplan–Meier plots depicting the use of antidepressants after a breast cancer diagnosis in the two groups, censoring at the time of attendance at the rehabilitation center. Both plots indicated that antidepressant use was different for the two groups. We therefore conducted secondary analyses to evaluate use of antidepressants after rehabilitation, in which we entered the rehabilitation program as a time-dependent covariate, including only the group that attended the program and who had not previously used antidepressants. In the secondary analyses women were followed from date of diagnosis of breast cancer until death, emigration, recurrence of breast cancer, new primary cancer, two years after attendance or December 31, 2007 whichever came first. Further the analyses were separated in time between the breast cancer diagnosis and the rehabilitation program into 0–1, 1–2, and ≥ 2 years and adjusted for covariates identified as significantly or borderline significantly associated with use of antidepressants after breast cancer in the full dataset.
Results
Although the matching was successful with regard to age (), date of surgery (p = 0.53) and protocol version (p = 0.14) the women who attended the Dallund Rehabilitation Centre differed significantly from the comparison group: they had a higher level of education, more were affiliated to the work market, but they had a lower average income and more were users of antidepressants before breast cancer. Fewer were living with a partner but more had young children living at home. Further, they had more tumor-positive axillary lymph nodes, more were treated by mastectomy, and more experienced relapse or a new primary cancer during follow-up ().
Table I. Descriptive characteristics of a cohort of 1 247 women and their breast cancers, who attended a course at a rehabilitation center (RCD) and a matched comparison group of 2 903 women who did not.
Of the 4 150 women in the study, 624 (15%) redeemed two or more prescriptions for antidepressants during the period between 1995 and one year before a diagnosis of breast cancer (mean follow-up, 7.0 years; ). After breast cancer diagnosis 1 020 (25%) used antidepressants (mean follow-up, 3.3 years; 5–95% range, 0.3–7 years; data not shown). Among women who were not prior users of antidepressants, 279 (27%) of the group attending rehabilitation and 322 (13%) of the comparison group became users of antidepressants after their breast cancer diagnosis (data not shown).
Among women who were not prior users of antidepressants, the diagnosis of a new primary cancer significantly increased the adjusted hazard ratio for antidepressant about three-fold (HR, 3.34; 95% CI, 1.50–7.76) as did a recurrence of breast cancer (HR, 2.56; 95% CI, 1.86–3.52) (). Number of tumor-positive axillary lymph nodes (HR, 1.01; 95% CI, 1.00–1.03 per node) were borderline significantly associated with use of antidepressants during follow-up (). No other clinical or treatment specific factors were associated with use of antidepressants. Being unemployed increased the hazard ratio for use of antidepressants (HR, 1.56; 95% CI, 1.16–2.10), whereas having children living at home and higher income (per 25 000 DKK) reduced the HR for use of antidepressants at borderline significance (HR, 0.79; 95% CI, 0.62–1.00; HR, 0.98; 95% CI, 0.96–1.00, respectively).
Table II. Adjusted hazard ratios for use of antidepressant medicationa after treatment for breast cancer, in women who did not use antidepressants before the diagnosis, mutually adjusted on all covariates. RCD attendees and comparison group, n = 3513.b
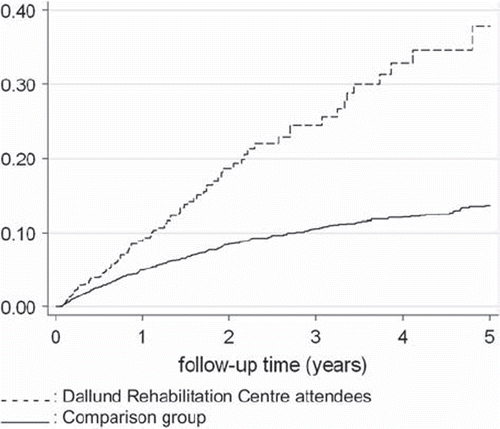
A marked difference in the rate of antidepressant use after diagnosis of breast cancer was observed between women attending rehabilitation (censored at the time of course attendance) and the comparison group, in both the unadjusted analysis () and after adjustment for covariates associated with use of antidepressants (data not shown) and this precluded direct comparison in the analyses of the effect of the rehabilitation stay and we performed secondary analyses.
Figure 1. Kaplan–Meier failure plots of use of antidepressants during the first five years after diagnosis of breast cancer, among women who were not prior users; Dallund Rehabilitation Centre attendees were censored from the plot at time of course attendance.
In the secondary analyses with the rehabilitation stay evaluated as a time-dependent covariate, the hazard ratio for use of antidepressants did not change after the rehabilitation stay for women who attended within the first year after breast cancer (HR, 1.02; 95% CI, 0.46–2.29), but nonsignificantly reduced hazard ratios were seen for women attending the rehabilitation stay within the second year or two or more years after breast cancer (HR, 0.44; 95% CI, 0.18–1.07 and HR, 0.69; 95% CI, 0.33–1.44, respectively; ).
Table III. Adjusted hazard ratios for use of antidepressant medicationa in 1 034b breast cancer patients who did not use antidepressant medication before diagnosis and who attended a rehabilitation program after breast cancer, by time between the breast cancer diagnosis and the rehabilitation program.
Discussion
In this study of breast cancer survivors who did not use antidepressant medications before their cancer disease, a recurrence of breast cancer and a new primary cancer were associated with increased rates of use of antidepressants whereas number of tumor-positive lymph nodes was associated only with borderline significance. Being unemployed and having lower income was associated with increased rates of use of antidepressants whereas having children living at home was associated with reduced rates, although the latter two associations were of borderline significance. Use of antidepressants was more frequent among women who attended the rehabilitation program, both before and, even more markedly, after their diagnosis of breast cancer, than in the comparison group. Although there was an indication of reduced use of antidepressants after attending the rehabilitation program, this finding must be interpreted with caution as we could not compare rates of antidepressant use with that of the comparison women.
The presented sample of well-characterized breast cancer patients, the well-described rehabilitation regime, and the use of prescription-based exposure information are unique. We included a matched sample of breast cancer patients and linked to national Danish registers to obtain unbiased, complete measures of pre-cancer sociodemographics as well as extensive information on disease and treatment characteristics.
The rates of prescription of antidepressant medication do not entirely reflect the rate of depression in a population, and, as we used register-based data, we cannot evaluate whether the antidepressants were prescribed in accordance with recommendations. Despite this limitation, the number of prescriptions of antidepressants largely reflects the rate of patients treated for affective disorders [Citation15,Citation16]; furthermore, register-based assessment of antidepressant use has been shown to be more exact than patients’ own recall [Citation23].
Use of antidepressants in the treatment of hot flushes, a known side-effect of antihormonal adjuvant treatment, has been suggested [Citation24]. To address this possible confounder, we contacted three of the major Danish breast cancer clinics (Herlev Hospital, Odense Universitetshospital, and Aarhus Sygehus) together treating approximately half of Danish breast cancer patients [Citation25]. In all three clinics, clonidin was the first choice for treating hot flushes, and antidepressants were prescribed only if clonidin did not have the desired effect. The pattern of a prescription for clonidin followed by a prescription for antidepressants within six months was observed for only 14 women in our data set, probably indicating that the problem is negligible.
Our finding that 25% of breast cancer patients used antidepressants at some time after diagnosis within the period of follow-up of up to seven years is in line with the reports of 19–36% users of antidepressants in two US studies [Citation13,Citation14].
Associations between income and education and depressive symptoms have been reported previously [Citation8,Citation9,Citation11]; we, however, found no association with education and an association of only borderline significance with income on use of antidepressants. We did observe that unemployment at the time of cancer diagnosis was associated with later use of antidepressants by patients who had not previously used them. While an American study found no significant association between unemployment and use of antidepressants in breast cancer survivors [Citation13], an association between depression and unemployment was described in a study of Danish breast cancer patients [Citation8]. Having children living at home was inversely associated with use of antidepressants, possibly indicating that the social network buffers a depressive reaction.
Our finding of an increased hazard ratio for use of antidepressants after a recurrence of breast cancer is in line with the result of another study, which found that recurrence is an independent risk factor for depression [Citation12]. The borderline significant association between the number of tumor positive lymph nodes and first use of antidepressants is in line with the finding of a recent Danish population-based study with a partly overlapping study population in which having more than three tumor-positive lymph nodes was the only cancer-related risk factor identified for depressive symptoms as measured by the Beck Depression Inventory among patients with newly diagnosed breast cancer [Citation8]. The number of tumor-positive axillary lymph nodes is the strongest prognostic factor in breast cancer; to the extent that this is recognized by women, it might result in more worry, depression, and use of antidepressants [Citation8]. Tumor-positive lymph nodes might result in more severe physical symptoms, a higher progression rate, and more radical treatment, all of which could lead to greater susceptibility to depressive symptoms.
The markedly larger proportion of users of antidepressants among the women attending the Dallund Rehabilitation Centre in comparison with the comparison group was unexpected. We speculate that the difference might indicate greater susceptibility to depressive symptoms, leading to more antidepressant prescriptions and an increased need for rehabilitation. Alternatively, our findings might identify a group of women with breast cancer who more readily obtain supportive care, which could lead to recognition of depression and prescription of antidepressants at an early time, as well as enrolment in a rehabilitation program. Whatever the cause, we were unable to evaluate the effect of the rehabilitation stay by comparing the two groups directly for use of antidepressant. Thus, in a secondary analysis, we made a comparison only within the group of women who attended rehabilitation. Furthermore, our study depicts the natural setting of the rehabilitation program for Danish breast cancer patients; we were unable to evaluate the program in a randomized setting. A trend to reduced use of antidepressants was seen among women who attended the rehabilitation center more than one year after breast cancer. We suggest that this might be due not to an increasing effect of the rehabilitation program with time after a breast cancer diagnosis but rather to prescription of antidepressants to more and more women susceptible to depression over time, leaving women less prone to use of antidepressants in the analysis.
In conclusion we found that recurrence of breast cancer and diagnosis of a new cancer considerably increased the rate of antidepressant use. Further sociodemographic rather than disease- or treatment-related characteristics at the time of diagnosis were associated with first use of antidepressants after a breast cancer diagnosis. Use of antidepressant medication was markedly higher, both before and after diagnosis of breast cancer, among the women who attended the rehabilitation program, indicating increased susceptibility to use of antidepressants by women who are referred.
Acknowledgements
The Danish Council for Independent Research/Medical Sciences supported the project with a scholarship to the main author Nis P. Suppli. The authors declare that they have no commercial or other associations that might pose a conflict of interest in connection with this article.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Bray F, McCarron P, Parkin DM. The changing global patterns of female breast cancer incidence and mortality. Breast Cancer Res 2004;6:229–39.
- Rowland JH, Hewitt M, Ganz PA. Cancer survivorship: A new challenge in delivering quality cancer care. J Clin Oncol 2006;24:5101–4.
- Fann JR, Thomas-Rich AM, Katon WJ, Cowley D, Pepping M, McGregor BA, . Major depression after breast cancer: A review of epidemiology and treatment. Gen Hosp Psychiatry 2008;30:112–26.
- Satin JR, Linden W, Phillips MJ. Depression as a predictor of disease progression and mortality in cancer patients: A meta-analysis. Cancer 2009;115:5349–61.
- DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: Meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med 2000;160:2101–7.
- Fallowfield L, Ratcliffe D, Jenkins V, Saul J. Psychiatric morbidity and its recognition by doctors in patients with cancer. Br J Cancer 2001;84:1011–5.
- Sharpe M, Strong V, Allen K, Rush R, Postma K, Tulloh A, . Major depression in outpatients attending a regional cancer centre: Screening and unmet treatment needs. Br J Cancer 2004;90:314–20.
- Christensen S, Zachariae R, Jensen AB, Vaeth M, Møller S, Ravnsbaek J, . Prevalence and risk of depressive symptoms 3-4 months post-surgery in a nationwide cohort study of Danish women treated for early stage breast-cancer. Breast Cancer Res Treat 2009;113:339–55.
- Kim SH, Son BH, Hwang SY, Han W, Yang JH, Lee S, . Fatigue and depression in disease-free breast cancer survivors: Prevalence, correlates, and association with quality of life. J Pain Symptom Manage 2008;35:644–55.
- Bardwell WA, Natarajan L, Dimsdale JE, Rock CL, Mortimer JE, Hollenbach K, . Objective cancer-related variables are not associated with depressive symptoms in women treated for early-stage breast cancer. J Clin Oncol 2006;24:2420–7.
- Osborne RH, Elsworth GR, Hopper JL. Age-specific norms and determinants of anxiety and depression in 731 women with breast cancer recruited through a population-based cancer registry. Eur J Cancer 2003;39:755–62.
- Burgess C, Cornelius V, Love S, Graham J, Richards M, Ramirez A. Depression and anxiety in women with early breast cancer: Five year observational cohort study. BMJ 2005;330:702.
- Azzone V, Frank RG, Pakes JR, Earle CC, Hassett MJ. Behavioral health services for women who have breast cancer. J Clin Oncol 2009;27:706–12.
- Ashbury FD, Madlensky L, Raich P, Thompson M, Whitney G, Hotz K, . Antidepressant prescribing in community cancer care. Support Care Cancer 2003;11:278–85.
- Gardarsdottir H, Heerdink ER, van DL, Egberts AC. Indications for antidepressant drug prescribing in general practice in the Netherlands. J Affect Disord 2007;98:109–15.
- Petty DR, House A, Knapp P, Raynor T, Zermansky A. Prevalence, duration and indications for prescribing of antidepressants in primary care. Age Ageing 2006;35:523–6.
- Hoybye MT, Dalton SO, Christensen J, Larsen LR, Kuhn KG, Jensen JN, . Research in Danish cancer rehabilitation: Social characteristics and late effects of cancer among participants in the FOCARE research project. Acta Oncol 2008;47:47–55.
- Moller S, Jensen MB, Ejlertsen B, Bjerre KD, Larsen M, Hansen HB, . The clinical database and the treatment guidelines of the Danish Breast Cancer Cooperative Group (DBCG); its 30-years experience and future promise. Acta Oncol 2008;47:506–24.
- Thygesen L. The register-based system of demographic and social statistics in Denmark. Stat J UN Econ Comm Eur 1995;12:49–55.
- Nickelsen TN. Data validity and coverage in the Danish National Health Registry. A literature review. Ugeskr Laeger 2001;164:33–7. Danish.
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 1987;40: 373–83.
- Gaist D, Sorensen HT, Hallas J. The Danish prescription registries. Dan Med Bull 1997;44:445–8.
- Boudreau DM, Daling JR, Malone KE, Gardner JS, Blough DK, Heckbert SR. A validation study of patient interview data and pharmacy records for antihypertensive, statin, and antidepressant medication use among older women. Am J Epidemiol 2004;159:308–17.
- Loprinzi CL, Wolf SL, Barton DL, Laack NN. Symptom management in premenopausal patients with breast cancer. Lancet Oncol 2008;9:993–1001.
- The Danish National Board of Health. [Internet]. Copenhagen (Denmark): Sundhedsstyrelsen; Kræftprofil - Brystkræft 2000–2007 [cited 2010 Mar]. Available from: http://www.sst.dk/publ/Publ2009/EPT/Kraeftprofiler/Kraeftprofil_Brystkraeft_2000-2007.pdf. Danish. 2009.