Abstract
Introduction. The aim of cancer rehabilitation is to enable patients to attain and maintain optimal physical, psychological and social functioning. We evaluated the effect on health behavior, BMI and self-rated health of a residential psychosocial rehabilitation course for cancer patients. Material and methods. Patients with a primary cancer of the breast, prostate, colon or rectum were randomized to either a six-day multi-focus psychosocial residential rehabilitation intervention that included lectures, discussions and peer group discussions on issues related to treatment and life with cancer or to usual care. The end points were changes in smoking, alcohol consumption, physical activity, body mass index and self-rated health between baseline and follow-up after one and six months. The primary analyses included all participants who received their allocated condition. The two follow-up times were analyzed separately in general linear and logistic regression models for continuous and dichotomous outcomes, respectively. The analyses were adjusted for baseline outcome score, cancer site, time since diagnosis, age and education. Results. Of the 507 participants who were randomly assigned, 452 were included in the analysis, of whom 404 completed the one month and 394 completed the six month assessment. The intervention group showed slightly more positive changes in health behavior, BMI and self-rated health than the usual care group, but the differences between the groups were small and not significant. Discussion. Participation in a six-day cancer rehabilitation course did not significantly influence health behavior, BMI or self-rated health among cancer patients.
Rehabilitation has been defined by the World Health Organization as a process aimed at enabling people with disabilities to reach and maintain their optimal physical, sensory, intellectual, psychological and social functional level [Citation1]. As more people survive their cancer and cancer can have severe effects, many cancer patients potentially need rehabilitation [Citation2]. Thus, it is important to identify effective methods to rehabilitate these patients. The aim of this study was to evaluate the effects of a psychosocial rehabilitation intervention on health behavior, BMI and self-rated health.
Previous studies have shown positive health effects in cancer patients who have improved their health behavior. For instance, smoking cessation appeared to decrease the risk for recurrence of cancer and for other smoking-related cancers. Consuming more fruit and vegetables; less saturated fat and alcohol may help adjustment to and survival from cancer [Citation3,Citation4]. In addition physical activity may be effective in relieving side-effects of cancer, such as mood swings, weight gain, sleep problems and fatigue [Citation4]; and improve physical functioning [Citation5] and well-being [Citation6].
Most intervention studies on changes in the health behavior of cancer patients have focused on a single behavior. Most evaluated the effects of dietary or physical training programs, and most were long-term, guided and intensive. Few studies have addressed smoking and alcohol consumption [Citation7,Citation8].
Self-rated health is considered a valid indicator of people's general health and has been shown to be a strong predictor of survival from cancer [Citation9]. The results of reviews and meta-analyses suggest that psychosocial interventions have beneficial effects on quality of life and psychological indicators, but the results are inconsistent [Citation10–12]. Self-rated health is a multidimensional construct, associated with illness-related factors (such as the presence of chronic disease), socio-demographic factors (e.g. age and income), health behavior (physical activity, smoking and alcohol habits) and psychosocial resources (such as social support) [Citation13]. We therefore hypothesized that self-rated health would improve after a rehabilitation intervention that focused on physical, psychological and social functioning.
Cancer rehabilitation became part of Danish health policy in 2000, when the national cancer plan explicitly described cancer as an illness with implications beyond medical treatment. In 2002, the Danish Cancer Society opened a cancer rehabilitation center. This study, with a randomized design, addressed the effects of a six-day residential psychosocial rehabilitation course on health behavior, BMI and self-rated health. We hypothesized that the psychosocial intervention would influence health behavior in a positive direction, i.e. reduce tobacco smoking and alcohol consumption, increase physical activity, decrease BMI and improve self-rated health.
Material and methods
Participants
Participants were recruited from six hospital departments for breast surgery, urology and gastrointestinal surgery in two Danish counties, Aarhus and Frederiksborg. Patients were eligible if they had had a primary cancer of the breast, prostate, colon or rectum, were diagnosed within the past two years, had completed primary treatment (except for hormonal treatment) and were able to participate physically in the intervention activities. Patients were excluded if they were in acute need of treatment or in terminal phase (estimated life expectancy, less than six months). No restrictions were made with respect to relapse or late effects.
Study design
The study was a randomized controlled trial of a rehabilitation intervention program compared with usual clinical care. At baseline and three follow-up times, patients filled out a questionnaire on self-reported measures of study protocol defined primary and secondary outcomes. In this paper we report the secondary outcomes of the study (health behavior, BMI and self-rated health) at baseline and at the one- and six-month follow-ups. The primary end points (distress and quality of life) will be reported in another paper [Citation14]. Data analyses from the 12 months follow-up will be reported separately.
Intervention. Participants randomized to the intervention group participated in a multi-focus psychosocial empowerment intervention at the Dallund Rehabilitation Centre, run by the Danish Cancer Society. Each week, 20 cancer patients were offered a six-day retreat, in which they participated with non-randomized patients from other counties. The overall aim of the intervention was to strengthen each individual's physical, psychological and social functioning. Sharing experiences with peers was a central aspect. The retreat consisted of a combination of lectures, discussions and patient group work on the treatment of cancer, psychological reactions, spirituality, sexuality, working life and lifestyle. The rehabilitation program included sessions on health behavior but was not explicitly designed to target specific health behavior changes. Appendix 1, can be found online at www.informahealthcare.com/10.3109/0284186X.2010.531761, describes a typical week program at the rehabilitation center. The usual care group received clinical control visits with standard follow-up regimens specific to their cancer site, but no systematic rehabilitation activities were offered. The intervention is described in detail elsewhere [Citation15].
Study objectives and outcomes. We evaluated the impact of the rehabilitation course on health behavior, BMI and self-rated health compared with usual care. We hypothesized that the intervention would influence health behavior in a positive direction, i.e. reduce tobacco smoking and alcohol consumption, increase physical activity, decrease BMI and improve self-rated health.
Socio-demographic and clinical information. A unique 10-digit personal identification number assigned to all residents of Denmark by the Central Population Register permits linkage of information between registers and precise information on age. Self-reported information on education and employment status was obtained from the baseline questionnaire. Cancer diagnosis, date of diagnosis, treatment modalities and tumor stage were obtained from nationwide clinical cancer databases for breast [Citation16] and colorectal cancer [Citation17] and from medical records for prostate cancer. If information was not available in these data sources, the information was retrieved from the Danish Cancer Registry, when possible [Citation18].
Sample size. When planning the study, we estimated that 600 patients should be included in the study. No formal power analysis was performed.
Randomization. Patients were randomly assigned to the intervention or usual care by use of a computerized random number generator operated through a secure website accessible by all participating departments. To ensure two groups of similar size, randomization was blocked into lengths of ten. The allocation sequence was generated by the study statistician, and other research staff was unaware of allocation details.
Patients were enrolled by the project coordinators from the hospital wards or the research department. Eligible patients were approached in person or by telephone and received written and oral information about the study if they were interested. Participants signed a document confirming their informed consent.
Once a patient had completed the baseline questionnaire, the project coordinators entered the patient's data into the study database via the secure website and a study number was automatically assigned. The patients were notified of their intervention status by the same project coordinator who gave them information about the study.
Statistical methods
Descriptive measures were used to examine demographic and disease-related variables and outcome values at baseline. The baseline characteristics of the intervention and usual care groups were compared in multiple linear or logistic regression models.
Primary analyses were conducted of data for all participants who received the condition to which they had been allocated. Data from the two follow-up times, one and six months, were analyzed separately, and persons who had not provided follow-up questionnaire, where excluded from the analyses of the specific follow-up time. Further, persons who had not responded to a single item were excluded from the analyses of that specific item, but not from analyses of other items. In order to evaluate the impact of the intervention fully, we investigated whether the changes in health behavior, BMI and self-rated health were different and also whether the magnitude of the changes was different between the intervention and the usual care group. Each outcome was therefore analyzed in three steps. First, we analyzed whether the mean changes in health behavior, BMI or self-rated health between baseline and follow-up differed significantly between the two groups. Secondly, we defined participants who had changed their health behavior, BMI or self-rated health in a positive direction and analyzed whether more people in the intervention than in the usual care group had changed positively. Thirdly, among a subgroup of people who had changed in a positive direction, we tested whether the positive change was greater in the intervention than in the usual care group.
We used linear regression models in the first step and logistic regression in the second. For the third step, we used linear regression models including only the positive direction change groups. All analyses were performed with assignment to intervention or usual care group as an independent variable. Due to the nearly 20% dropout from the intervention group analyses were adjusted for the baseline score of the outcome variable, cancer site, time since diagnosis, gender, age at baseline and educational level.
For the linear models, normal distribution was tested graphically for each outcome and could be assumed. The linearity of age, time since diagnosis and baseline score of the outcome was evaluated on each outcome in linear splines with knots placed at the quartiles. The statistical analyses were conducted with SAS version 9.1.
Calculation of outcomes. Single outcome values were calculated from one or several items of the outcome variable. Persons who reported extreme outcome values were excluded from analysis of that specific outcome to minimize the risk of information bias.
Total smoking per day was assessed from two questions ‘Do you smoke?’ and ‘How much do you smoke per day?’ and was calculated as the sum of grams of tobacco (from reported numbers of cigarettes, cheroots, cigars or pipes smoked per day). Three participants were excluded from the analysis as they reported smoking more than 80 g/day. A positive direction change was defined as changing from smoker to non-smoker between baseline and follow-up, as only total smoking cessation was considered a relevant behavior change, and also what the National Board of Health in Denmark recommend.
The total intake of alcohol/week was assessed from one question: ‘How many units of alcohol did you consume on each of the week days last week?’ and was calculated as the sum of alcohol units/day. Abstainers (n=62) and participants who reported drinking more than 35 units/week (n=9) were excluded from the analysis. This was due to the fact that most people in Denmark do consume alcohol and abstainers may thus be considered a special group of people according to alcohol behavior who might not respond to the intervention as expected. A positive direction change was defined as a decrease in alcohol consumption of more than 2 units/week.
Total hours of physical activity/week was assessed from one question: ‘Looking back on the last month, how many hours did you on average use per week on physical activity?’ and were calculated as the sum of hours spent in six activity categories: walking, bicycling, home activities (e.g. cleaning, shopping), home repair, gardening and sports. Participants who reported more than 49 hours of physical activity/week were excluded from the analysis (n=42). A positive direction change was defined as an increase of more than 3.5 h/week, as most of the activities were not vigorous.
Total hours of sport/week were assessed from the physical activity question only summing hours of activities from sports (gymnastics, running, swimming, etc.). A positive direction change was defined as an increase of more than one h/week. Sport was analyzed separately from total physical activity, because the intensity during sport is normally higher than during other activities. In addition, previous studies have shown that more structured activities are reported in a more valid manner than other activities [Citation19].
BMI was assessed from two questions ‘What is your height?’ and ‘What is your weight?’ and was calculated as weight/height2. People were excluded from the analysis if their height differed by more than 5 cm (n=12), if their weight differed by more than 10 kg (n=5) and 30 kg (n=3) between baseline and follow-up one and six month, respectively, or if their BMI was lower than 18.5 (n=3) or higher than 50 (n=2). A positive direction change was defined as a more than 2.5% decrease in BMI.
Self-rated health was assessed from one question: ‘In general, would you say your health is excellent, very good, good, fair or poor’ and measured on a scale from 1 (poor) to 5 (excellent) [Citation20]. A positive change was defined as any positive change on the scale.
Sensitivity analysis. To test whether chosen cut-offs for positive direction changes were defined reasonably, we performed sensitivity analyses. We considered any positive change between baseline and follow-up as a positive change; e.g. a positive direction with regard to BMI was defined as any decrease.
We could not perform intention to treat analyses, as, owing to a system error, follow-up questionnaires were not sent to patients who did not participate in the intervention as scheduled. To test what the results might have been, had the 51 non-receivers attended at the intervention, we performed additional analysis based on three scenarios, setting behavior change values in non-receivers at the 25th, 50th and the 75th percentile of that of the group who received the intervention. This was only done for first step analyses and for relevant outcomes.
Results
Study population
Between May 2004 and September 2008, 507 patients were randomized, with 259 patients in the intervention and 248 in the usual care group (). No records were kept of people who did not meet the eligibility criteria or of those who refused to participate. The intervention group contained more patients with breast cancer and fewer with prostate cancer (p=0.04), fewer men (p=0.01) and more current smokers (p=0.05) than the usual care group. No other significant differences were observed (p-values not shown) ().
Figure 1. Randomization and follow-up of cancer patients eligible for participation in the randomized controlled trial of psychosocial cancer rehabilitation in Denmark, 2004–2008. 1Baseline assessment before randomization, 2Includes illness in family, lack of energy to participate or emigration, 3One participant attended six-month follow-up, 4Six participants attended six-month follow-up.
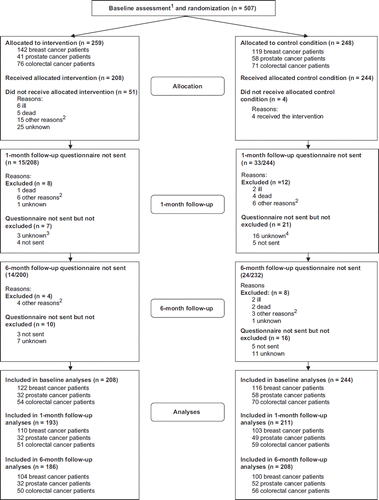
Table I. Baseline characteristics of 507 participants with breast, prostate or colorectal cancer, by allocated intervention status.
Exclusion of 55 (11%) participants who did not receive the allocated condition left 452 participants for analysis. In comparison with those who received the intervention, those who did not receive it comprised fewer patients with breast cancer (p=0.01) and more with colorectal cancer (p=0.01) ().
Further exclusions are illustrated in . In all, 452 (89%) provided the baseline questionnaire, 404 (80%) provided the one month follow-up and 394 (78%) the six month follow-up questionnaire.
Primary analyses
Overall, the changes in health behavior, BMI and self-rated health between baseline and follow-up at one and six months were not significantly different between the intervention and the usual care group ( and ). For most outcomes, the mean changes were more positive () and more people showed positive changes () in the intervention than the usual care group, but the differences were small and not significant. Only for physical activity there was a tendency (not significant) to increased activity (p=0.07) () and more people increased their activity (p= 0.09) () at six months in the intervention group.
Table II. Changes in health behavior, BMI and self-rated health: mean differences between baseline and follow-up.
Table III. Changes in health behavior, BMI and self-rated health: proportions with positive change between baseline and follow-up.
Subgroup analyses
Among participants whose BMI decreased, the decrease was greater in the intervention group than in the usual care group (p=0.04, six-month follow-up). In the subgroup who decreased their alcohol intake, the decrease was larger in the intervention than in the usual care group (p=0.04, one-month follow-up). For the other outcomes, there were no significant differences between subgroups (). Further characteristics of the groups that changed positively are provided in Appendix 2, can be found online at www.informahealthcare.com/10.3109/0284186X.2010.531761.
Table IV. Changes in health behavior, BMI and self-rated health: mean differences between baseline and follow-up in subgroups with positive change.
Sensitivity analyses
The results of the sensitivity analysis showed no overall differences from the main and subgroup analyses. There were significant differences between the intervention and usual care groups in the subgroup analysis of BMI (p=0.04 at one-month follow-up and p=0.01 at six-month follow-up) and alcohol consumption (p=0.02 at one-month follow-up).
Including intervention non-receivers in the analyses of physical activity and BMI, revealed results close to the main analysis in 50th percentiles scenario (physical activity p=0.06 and BMI p=0.53 at six-months follow-up) and also non-significant results in the 25th percentile scenario. In the 75th percentile scenario the effect was significantly larger in the intervention than the usual care group (p<0.001) for physical activity and borderline significant for BMI (p=0.08) at six-months follow-up.
Discussion
This randomized study provides no convincing evidence of an effect on health behavior or BMI and no effect at all on self-rated health of a six-day rehabilitation intervention retreat.
The subgroup result for BMI is not regarded as a solid effect of the intervention, as the number of persons, whose BMI decreased was small and the actual decreases were small. Unfortunately, we had no data on dietary behavior. Other randomized and controlled studies of rehabilitation interventions in cancer patients have to a greater extent found effects on bodyweight or BMI and physical activity [Citation7,Citation8]. In a large study designed to change multiple health behavior, Social Cognitive Theory was used to motivate 3 031 families with cancer to adopt six cancer-prevention behaviors, and the patient's stages of change were identified before four tailored face-to-face sessions. The authors reported significant decreases in adverse health behavior (smoking, drinking, diet, weight and sun) in the intervention vs. control group after 18 months follow-up [Citation21].
One recent intervention study of diet and exercise found an effect on BMI of a 10-months written program (tailored with regard to, e.g. barriers, stage of readiness) in 543 patients with breast or prostate cancer after 12 months [Citation22]. A further three studies reported from dietary interventions in breast cancer patients [Citation23–25]. In one (n=2 437) an eating plan was introduced in eight biweekly (up to four months) individual counseling sessions, with behavioral, cognitive and motivational techniques (follow-up after five years) [Citation23]. Another study, of 85 overweight patients, incorporated elements of cognitive behavioral therapy in group sessions, including 16 weeks of advice to keep food diaries, exercise logs and records of physical exercises, four follow-up phone calls (follow-up after 16 weeks) [Citation24]. The third study (n=3 088) included a telephone counseling program (on average, 18 calls per patient), supplemented by 4–12 cooking classes, newsletters (follow-up after four years) [Citation25]. Two of these studies found small effects on weight or BMI [Citation23,Citation24] and one observed no differences in weight between groups [Citation25]. In a review of 11 randomized trials of dietary interventions (n=48–2 970) for women with breast cancer, significant improvements in body weight were found in all but one of the eight studies reporting on this outcome. Most of the interventions were intensive and included individual counseling by trained nutritionists, although positive findings were also made in three studies of less intensive interventions [Citation8]. Compared to our study, these dietary interventions were all focused on changes in a single health behavior, involved relatively homogeneous groups, were based on either individually tailored programs, behavioral theory or included follow-up boosters, and many were of long duration. In general, the changes in weight or BMI reported were rather small, but the differences between the intervention and control groups were larger than in our study, and they were based on whole samples rather than the subsample in our study.
We found no convincing effect on physical activity. In effective interventions, supervised guidance and specific training programs were used. Nine of 13 physical training interventions reviewed by Demark-Wahnefried et al. included more than 50 patients, and seven of these studies (n=53–450) of patients with breast and prostate cancer or cancers at various sites showed significant effects on physiological end points such as oxygen up-take and physical status [Citation8]. A review of 24 studies (10 not controlled) (n=10–150) showed an overall effect of 3–24-week resistance training programs with 2–3 sessions per week on peak oxygen uptake (increase, 6–39%) and maximum one-repetition capacity (increase, 11–110%) [Citation7].
The effect on alcohol intake in our study was not a maintained change, as it was significant only at the one-month follow-up in a subgroup. Likewise, in their study of a telephone intervention with tailored materials to 1 247 colorectal cancer patients, Emmons et al. found no effect on alcohol consumption when this behavior was analyzed separately from the targeted adverse health behavior measures (diet, alcohol, smoking and physical activity) [Citation26], while López et al. did find a separate effect on alcohol use in their multitargeted intervention [Citation22].
Demark-Wahnefried et al. reported no effect on smoking behavior in two of four intervention studies. In one study of 435 patients with breast, prostate or testicular cancer or lymphoma, a very brief (< 5 min) physician-delivered intervention on the benefits of quitting, nicotine replacement therapy, provision of materials and/or referral to a cessation program was evaluated. The other study of 186 patients with head-and-neck cancer, included surgeon-delivered cessation counseling after surgery, a booster session after six months (follow-up after 12 months) [Citation8]. The other two studies we considered too small (n=26 and 28, respectively [Citation8]) for conclusions to be drawn.
Our study showed no effect of the intervention on self-rated health. As this measure is considered to be an overall construct, including aspects of health behavior and psychosocial well-being, one would expect that changes in these outcomes could act as mediating steps to changed self-rated health. As no changes were found in these aspects in the study reported here or the other report from this trial on distress and quality of life aspects [Citation14], no effect on self-rated health is consistent with the intervention effect on these other study outcomes.
The strengths of this study include its randomized design and the large sample of cancer patients included after the end of primary treatment.
One limitation is the relatively large proportion (nearly 20%) of people randomized to the intervention who did not receive it, which compromises the internal validity. The baseline characteristics of the drop outs did not, however, result in substantial differences between the intervention and usual care groups. In order to compensate, the analyses were adjusted for relevant factors. We could not conduct intention-to-treat analyses, because the dropouts were not sent follow-up questionnaires. However, additional scenario analysis for physical activity and BMI showed that only if all non-receivers had changed positive to the 75th percentile of the intervention receivers, an effect of the intervention would have appeared. Based on the reasons for drop out in the non-receivers this scenario however, seems unlikely.
Another limitation is that we do not know whether our study population is representative of all eligible patients, as the number of patients assessed for eligibility is unknown; however we consider this to be a matter mainly of external validity [Citation27]. Lastly, the health behavior and BMI changes were self-reported, and no objective measures were used. There was no indication, however, that the intervention participants reported more positive results than usual care participants when filling in the questionnaires.
In contrast to many other types of rehabilitation investigated, the intervention evaluated in this study was delivered to a heterogeneous group of participants (in terms of cancer diagnosis, stage and individual problems), the duration was short, and many issues were introduced, allowing participants to choose those that seemed relevant to them. Professional identification of each patient's needs from baseline reporting of distress, health behavior or BMI and tailoring the intervention in terms of those needs might have made the intervention more effective. This is partly supported by the characteristics of intervention participants that improved their behavior or self-rated health. Those that lowered their alcohol intake had higher baseline alcohol intake, those increasing physical activity had lower baseline activity level, and those improving self-rated health had a lower score at baseline than did the intervention group as a whole although not formally tested. Use of more explicitly described theories of behavioral change might have focused the content of the intervention and further directed the outcomes of the evaluation. We report here, however, on a real-life intervention designed to provide cancer patients with information on how to move on with their lives. It was therefore not specifically focused on a single behavioral or psychosocial outcome [Citation15]. Although this is a limitation, it is often a premise when conducting research in a real-life setting.
In conclusion, we found no marked effect of a psychosocial cancer rehabilitation program on health behavior, BMI or self-rated health. Identification of individual problems and use of theories of behavioral changes might strengthen the impact of future rehabilitation interventions.
Supplementary Material
Download PDF (124.7 KB)Acknowledgments
This study was funded by the Danish Cancer Society. We wish to thank the cancer patients who participated in the study for their valuable contributions. We also wish to acknowledge the important collaboration of the staff at the Dallund Rehabilitation Centre and the participating hospital departments. The clinical trial registration number was Clinical Trials.gov protocol ID NCT01086683.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Who.int [Internet]. Geneva: World Health Organization [cited 2010 Jul 28]. Available from: http://www.who.int.
- Stein KD, Syrjala KL, Andrykowski MA. Physical and psychological long-term and late effects of cancer. Cancer 2008;112(11 Suppl):2577–92.
- Khan N, Afaq F, Mukhtar H. Lifestyle as risk factor for cancer: Evidence from human studies. Cancer Lett 2010;293:133–43.
- Kuhn KG, Boesen E, Ross L, Johansen C. Evaluation and outcome of behavioural changes in the rehabilitation of cancer patients: A review. Eur J Cancer 2005;41:216–24.
- Spence RR, Heesch KC, Brown WJ. Exercise and cancer rehabilitation: A systematic review. Cancer Treat Rev 2010;36:185–94.
- Knols R, Aaronson NK, Uebelhart D, Fransen J, Aufdemkampe G. Physical exercise in cancer patients during and after medical treatment: A systematic review of randomized and controlled clinical trials. J Clin Oncol 2005;23:3830–42.
- De Backer I, Schep G, Backx FJ, Vreugdenhil G, Kuipers H. Resistance training in cancer survivors: A systematic review. Int J Sports Med 2009;30:703–12.
- Demark-Wahnefried W, Aziz NM, Rowland JH, Pinto BM. Riding the crest of the teachable moment: Promoting long-term health after the diagnosis of cancer. J Clin Oncol 2005;23:5814–30.
- Shadbolt B, Barresi J, Craft P. Self-rated health as a predictor of survival among patients with advanced cancer. J Clin Oncol 2002;20:2514–9.
- Jacobsen PB, Jim HS. Psychosocial interventions for anxiety and depression in adult cancer patients: Achievements and challenges. CA Cancer J Clin 2008;58:214–30.
- Osborn RL, Demoncada AC, Feuerstein M. Psychosocial interventions for depression, anxiety, and quality of life in cancer survivors: Meta-analyses. Int J Psychiatry Med 2006; 36:13–34.
- Ross L, Boesen EH, Dalton SO, Johansen C. Mind and cancer: Does psychosocial intervention improve survival and psychological well-being? Eur J Cancer 2002;38: 1447–57.
- Cott CA, Gignac MA, Badley EM. Determinants of self rated health for Canadians with chronic disease and disability. J Epidemiol Community Health 1999;53:731–6.
- Rottmann N, Dalton SO, Würtzen H, Christensen J, Hansen DG, Bidstrup PE, . Does psychosocial rehabilitation course relieve distress and improve quality of life in cancer patients? – A randomized controlled trial. 2010 (in press).
- Hoybye MT, Dalton SO, Christensen J, Larsen LR, Kuhn KG, Jensen JN, . Research in Danish cancer rehabilitation: Social characteristics and late effects of cancer among participants in the FOCARE research project. Acta Oncol 2008;47:47–55.
- Moller S, Jensen MB, Ejlertsen B, Bjerre KD, Larsen M, Hansen HB, . The clinical database and the treatment guidelines of the Danish Breast Cancer Cooperative Group (DBCG); its 30-years experience and future promise. Acta Oncol 2008;47:506–24.
- Harling H, Nickelsen T. The Danish Colorectal Cancer Database. Ugeskr Laeger 2005;167:4187–9. Danish.
- Storm HH, Michelsen EV, Clemmensen IH, Pihl J. The Danish Cancer Registry – history, content, quality and use. Dan Med Bull 1997;44:535–9.
- Richardson MT, Ainsworth BE, Wu HC, Jacobs DR, Jr., Leon AS. Ability of the Atherosclerosis Risk in Communities (ARIC)/Baecke Questionnaire to assess leisure-time physical activity. Int J Epidemiol 1995;24:685–93.
- Ware JE, Kosinski M, Keller SK. SF-36. Physical and mental health summary scales: A user's manual. Boston, MA: The Health Institute; 1994.
- Lopez ML, Iglesias JM, del Valle MO, Comas A, Fernandez JM, de VH, . Impact of a primary care intervention on smoking, drinking, diet, weight, sun exposure, and work risk in families with cancer experience. Cancer Causes Control 2007;18:525–35.
- Demark-Wahnefried W, Clipp EC, Lipkus IM, Lobach D, Snyder DC, Sloane R, . Main outcomes of the FRESH START trial: A sequentially tailored, diet and exercise mailed print intervention among breast and prostate cancer survivors. J Clin Oncol 2007;25:2709–18.
- Hoy MK, Winters BL, Chlebowski RT, Papoutsakis C, Shapiro A, Lubin MP, . Implementing a low-fat eating plan in the Women's Intervention Nutrition Study. J Am Diet Assoc 2009;109:688–96.
- Pierce JP, Natarajan L, Caan BJ, Parker BA, Greenberg ER, Flatt SW, . Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: The Women's Healthy Eating and Living (WHEL) randomized trial. JAMA 2007;298:289–98.
- Mefferd K, Nichols JF, Pakiz B, Rock CL. A cognitive behavioral therapy intervention to promote weight loss improves body composition and blood lipid profiles among overweight breast cancer survivors. Breast Cancer Res Treat 2007;104: 145–52.
- Emmons KM, McBride CM, Puleo E, Pollak KI, Clipp E, Kuntz K, . Project PREVENT: A randomized trial to reduce multiple behavioral risk factors for colon cancer. Cancer Epidemiol Biomarkers Prev 2005;14:1453–9.
- Meinert CL. Beyond CONSORT: Need for improved reporting standards for clinical trials. Consolidated Standards of Reporting Trials. JAMA 1998;279:1487–9.