Abstract
Background. The effect of interventions that support rehabilitation among cancer patients has to be tested before implementation. Objective. A randomised controlled trial was conducted to test the hypothesis that a multimodal intervention may give the general practitioner (GP) an enhanced role and improve rehabilitation for cancer patients. The intervention included an interview about rehabilitation needs with a rehabilitation coordinator (RC), information from the hospital to the general practitioner about individual needs for rehabilitation and an incentive for the GP to contact the patient about rehabilitation. The objective of this first report from the study was to examine the acceptability and feasibility of the intervention. Material and methods. Adult patients treated for incident cancer at Vejle Hospital, Denmark were included between May 12, 2008 and February 28, 2009. All general practices in Denmark were randomised. Patients were allocated to intervention or control (usual procedures) based on the randomisation status of their GP. The feasibility of the intervention was analysed with regard to recruitment of patients, acceptability by patients and GPs and the degree to which the planned contacts between patients, RCs and GPs were implemented. The primary outcome of the randomised controlled trial (RCT) will be health-related quality of life at six months (EORTC-30). Results. Following assessment of 1 896 cancer patients, 955 patients (50%) registered with 323 general practices were included. The interview was conducted at the hospital with 50% of the patients in the intervention group, 31% were contacted by phone. Patients valued the fact that the conversation was dedicated to needs beyond the medical treatment. The GPs were generally available for information by phone and positive towards having a central role in the cancer rehabilitation. Discussion. It was feasible to conduct a RCT to evaluate a complex intervention in the healthcare system. All elements of the intervention were acceptable and feasible and may be implemented in future practice if the effect is positive.
There is increased focus on cancer patients’ unfulfilled needs for individual rehabilitation taking into consideration the physical, psychological, social, economic, as well as work-related consequences of the cancer disease [Citation1,Citation2]. Patients experience various significant problems as well as lack of professional support [Citation3]. Particularly the psychological and social problems arising in the wake of the disease are neglected in preference of problems related to treatment and side-effects [Citation4,Citation5].
It is an international challenge to determine a well-founded and systematic approach to accommodate the diverse rehabilitation needs [Citation6–8]. There is a great need for research testing new ways to organise the rehabilitation services across different settings [Citation5,Citation9,Citation10]. Research may contribute to the improvement of the quality, the organisation and procurement of the rehabilitation offers as well as the collaboration and communication about each patient's rehabilitation course within and across healthcare sectors. These improvements will presumably lead to patients experiencing greater satisfaction with their rehabilitation and enhanced quality of life.
The general practitioner (GP) is often the most consistent professional person before, during and after the hospital-based treatment phase and may be the professional who has most knowledge about the patient's life, health status prior to cancer, social network, and mental vulnerability. The needs for rehabilitation may occur from the time of diagnosis to long after the treatment and the regular contact to the hospital is completed [Citation11,Citation12], which supports the GP playing a central role in rehabilitation [Citation10,Citation13–15]. It is essential that the GP is made aware of the needs as they occur and also makes use of the opportunity to be proactive and outreaching to the patients [Citation16].
According to standard routines, the GP is updated on the patient's course of disease through discharge summaries. These comprise information about treatment, medication, investigations and sometimes a plan for future treatment and follow-up. Information on rehabilitation needs is usually neither identified nor included. In order to pass on the role as facilitator of rehabilitation to the GP, the process might be optimised if the concept of rehabilitation is introduced to the patients early after diagnosis and if their different needs are identified during hospitalisation and communicated to the GP along with the discharge summary. Proactive initiatives to further action from the GP's part might then optimise the course of rehabilitation for patients in general and accommodate those patients lacking the reserves of strength to turn to general practice or anywhere else for support [Citation14,Citation17].
A randomised controlled trial (RCT) was designed to test the hypothesis that a multimodal intervention may give the GP an enhanced role and improve rehabilitation for cancer patients. The complex intervention included a consultation about rehabilitation needs with a rehabilitation nurse (the RC), information from the hospital to the GP about individual needs for rehabilitation and an encouragement for the GP to contact the patient about rehabilitation.
The objective of this first report from the study was to examine the acceptability and feasibility of the intervention.
Material and methods
We designed a cluster randomised, controlled trial of GP involvement in cancer rehabilitation. All general practice units in Denmark were randomised to the intervention group (facilitation of an enhanced role for the GP in rehabilitation) or the control group (routine care). Patients were subsequently allocated according to the randomisation of their GP. The study protocol was described in accordance with the CONSORT statement [Citation18].
Participants
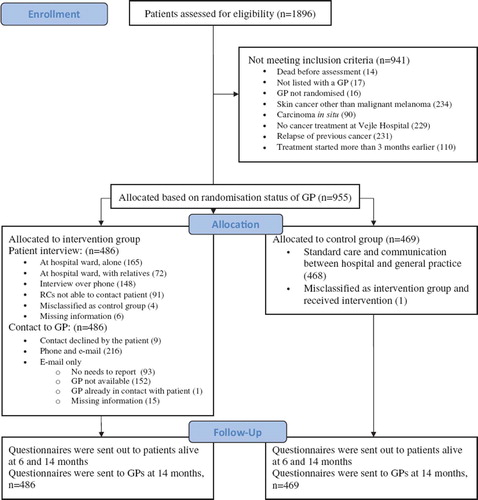
All adult patients (≥18 years) admitted to Vejle Hospital between May 12, 2008 and February 28, 2009 diagnosed with the AZCA-1 code: new cancer disorder not formerly registered by the department, were assessed for eligibility. Patients were included if treated at Vejle Hospital for a cancer diagnosed within the last three months and listed with a general practice randomised prior to study start. Patients with carcinoma in situ or non-melanoma skin cancers were not included ().
Two RCs who were nurses with oncological experience worked full-time to assess all patients and manage the intervention. The patients were sampled across departments, type of cancer, stage, and potential rehabilitation needs. Every second week all patients classified with the AZCA-1 code in the electronic patient files were listed and assessed for eligibility by review of electronic patient files. The study was conducted at all clinical departments at Vejle Hospital, and general practices across the country. Vejle Hospital is a public general hospital located in the region of Southern Denmark (1.2 million inhabitants) [Citation19] and one of two regional hospitals with oncological specialty. Vejle Hospital is characterised by integrated cancer diagnostic pathways and fast dissemination of discharge summaries to general practice.
The Danish publicly funded healthcare system ensures free access to general practice, in- and outpatients care for all citizens. More than 98% of all Danish residents are registered with a general practice. Each practice has a provider number in order to get remuneration from the National Health Service. Single-handed practices constitute 60%, 19% are shared by two GPs, 11% by three GPs and 9% by more than three GPs [Citation20]. The average number of patients per GP is 1 600. On average each GP meets nine incident cancer patients during one year.
The intervention
The intervention consisted of a targeted patient interview and information from the RC to the GP about the patient's individual rehabilitation needs and general information about rehabilitation. The general information comprised encouragement of the GP to contact the patient and take a proactive role as facilitator for the rehabilitation process. All GPs received written communication and were contacted by telephone.
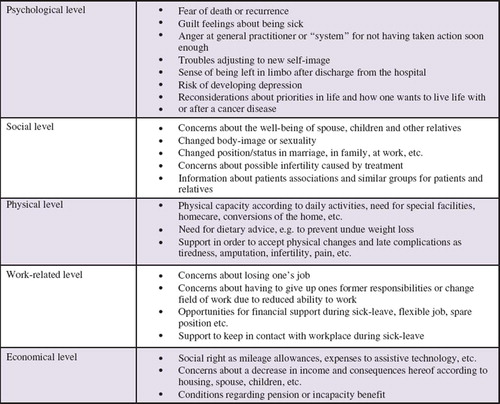
Patient interviews were conducted up to three months after inclusion and most often at the hospital ward or in the outpatient clinic on days the patient were scheduled for treatment (). For practical reasons the interview was in some cases conducted by phone with the patient at home. During the interview the concept of rehabilitation was introduced to the patient and individual needs for physical, psychological, social, work- and economy-related rehabilitation were identified. An interview manual including potential needs among cancer patients () was developed. It was explained to the patient that the personal consequences of cancer are complex and individual combinations of physical, psychological, sexual, social, work-related and economical issues [Citation3,Citation21] might occur at any time and probably change during the disease trajectory. In order to address the needs, if and when they occur, it was suggested to use the GP during treatment and especially after discharge. The structure of the interview was inspired by the method of the Calgary-Cambridge guide to the medical interview [Citation22]. With a systematic approach primarily open-ended questions were used during the 10–40 minute interview (average 15 minutes).
Following the patient interview the patient's GP was contacted by phone by the RC. This personal communication included the patient's actual problems and needs for rehabilitation and a request to act proactively towards rehabilitation of the specific patient, i.e. the GP was encouraged to call the patient to offer support and guidance. The telephone communication was followed up with an e-mail repeating the individual information and the request to contact the patient, supplemented by general information about needs and problems among cancer patients (). If additional attempts to get in personal contact with the GP failed, the flow of information only consisted of the personal e-mail.
Patients in the control group were not contacted by the RCs and received the usual care. Rehabilitation may have been discussed, but in the normal clinical context with no specific or systematic focus. GPs in the control group did not receive individual or general information about rehabilitation and the communication between the healthcare sectors was unchanged. Actual problems and needs may have been mentioned in discharge summaries, but this is rare.
Patient status (intervention or control) and problems and needs for rehabilitation were not added to patient files.
Outcomes and sampling of data
The effect of the intervention is to be evaluated with respect to health-related quality of life, the GP's behaviour (proactive or wait-and-see), patient satisfaction with the rehabilitation provided by the healthcare system in general and the GP in particular, and number of working days lost because of sickness leave.
The primary outcome of the randomised study will be patient-perceived health-related quality of life measured by the Global Health Status items at the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire C30 (EORTC) six months after diagnosis [Citation23,Citation24].
Secondary outcomes will include health-related quality of life (EORTC) at 14 months, additional subscales of the EORTC at six and 14 months, patient experienced psychological distress measured by Profile Of Mood States-Short Form (POMS-SF) [Citation25], number of working days lost to sickness, patient satisfaction with the GP (Dan-PEP) [Citation26] and evaluation of the GP's contribution to rehabilitation estimated by ad hoc questions to patients as well as GPs.
Data have been sampled in identical ways irrespective of allocation status by use of patient and GP questionnaires sent out at six and 14 months after inclusion to patients and at 14 months to GPs. Register data are to be obtained on diagnosis, disease stage, number of sick days, marital status, education and occupational status.
A six-month patient questionnaire with 134 items (11 pages) included the EORTC and Dan-PEP scales, questions about diagnostic delay, communication between healthcare sectors, degree of coherence in the course of cancer. A 14-month patient questionnaire with 171 items (15 pages) included five validated instruments (EORTC, POMS-SF, Dan-PEP, the Multidimensional Health Locus of Control (MHLC) scale Form B [Citation27] and part of the FACIT-sp questionnaire [Citation28] concerning religious and spiritual beliefs), and items about rehabilitation needs (somatic, psychological, social and occupational), how and where these needs were addressed, satisfaction with the rehabilitation provided by the healthcare system in general and the GP in particular, and social support. A 13-item GP questionnaire (two pages) addressed the GP's satisfaction with own contribution to the course of rehabilitation and cooperation with hospital and municipality about the individual patient.
Addresses and vital status were updated from the Central Population Registry prior to distribution of patient questionnaires. Non-responders were sent one reminder (questionnaire and pre-paid envelope) after three weeks. GP questionnaires were labelled with the name and personal registration number of the patient concerned. Non-responders were reminded three weeks later. A number of GPs were randomly selected to receive a small gift for their participation. The feasibility of the intervention was analysed with regard to identification of patients, acceptability of the intervention by patients and general practitioners and the degree of completion of the intervention. Information on percentage of patients available for interview and GPs available for personal contact were registered by the RCs together with a grading of the GP's immediate response to the contact.
Sample size
The sample size was estimated based on the primary outcome measure. According to the EORTC Tables of Reference Values for all cancer patients, all stages, the Global Health Status is normally distributed with a mean of 61.3 and a SD of 24.2. A change of 8 units was assumed to be clinically relevant.
If the lowest acceptable power was 80%, then, based on the two-sample t-test with a type 1 error alpha=0.05 and a type 2 error beta=0.2, the sample size was calculated to be 144 per group. The study was subject to clustering because the unit of randomisation was at the level of the GP, whereas the primary outcome measure was at the level of the patient. We did not have any data to estimate the effect of clustering prior to the study, but expected a strong effect on outcome of the practice patterns of individual GPs. It was therefore attempted to include patients in each arm of the trial from a minimum of 144 practice units (allowing for maximum clustering).
Randomisation
Prior to study start, all general practices in Denmark were randomised to an intervention or a control group. GPs working in the same practice (same provider number) were allocated together. The procedure was done by means of a computerised random-number generator.
Patients included were allocated by the RCs based on the randomisation status of the general practice with which the patient was registered.
Blinding
The study was not blinded. The list of randomisation was available to the RCs during assessment of patient eligibility and allocation status was obvious during intervention.
Statistical analysis: The effect of the intervention
Numerical outcomes of the RCT will be analysed using a mixed-effect linear model, accounting for cluster effects. Binary outcomes will be analysed using mixed-effect logistic regression, accounting for cluster effects. The analyses will adjust for potential confounders such as age, gender, cancer type and stage, and various GP characteristics when relevant. A specific focus will be on the association between patient gender and study outcome.
Ethics
The study was approved by the Danish Data Protection Agency and the medical chief executive officer of Vejle Hospital, Hospital Little Belt.
The Regional Committee on Biomedical Research Ethics evaluated the project and concluded that the intervention is considered to be an administrative procedure and therefore does not need approval as a biomedical trial.
Development and piloting of questionnaires and intervention
As background for designing the intervention we established a theoretical basis through review of papers, reports and textbooks about the problems faced by cancer patients and GPs with respect to individual rehabilitation and continuity across healthcare sectors [Citation3,Citation13,Citation21].
The three questionnaires were pilot tested during a three-step procedure including additional revision. Firstly, researchers were asked to comment on content, layout, volume and intelligibility of the draft. Secondly, small groups of cancer patients (n= 10) and general practitioners (n= 4) were asked to fill in the questionnaire under observation of a researcher and comment on content, layout, volume and intelligibility. Thirdly, large groups (350 patients, 100 patients and 40 GPs, respectively) were asked to fill in a mailed version enabling us to examine discrimination and acceptability and make the last corrections.
The procedures of identification, assessment and inclusion of patients were pilot tested by enrolment of 15 patients prior to study start. The AZCA-1 code was chosen as the determining tool of identification based on considerations of feasibility and completeness. The code may, however, be missing in some cases due to irregular use. The interview guide, communication procedure, written information to the GPs, logistics and data administration were also tested on these patients and minor corrections decided. During the pilot phase we learned the importance of clearly communicating to the GPs that the directions for contact and future role were proposals with constructive suggestions and not demands. The wording of the written communication was changed accordingly.
Results
A total of 1 896 patients received the AZCA-1 code and were eligible for assessment by the RCs. Approximately half of them, 955, fulfilled the criteria for inclusion (). The included patients pertained to 323 practice units (intervention group 164, control group 159). The most frequent types of cancers were breast (44%), gastrointestinal (15%), lung (15%), malignant melanoma (8%) and gynaecological (5%).
It was possible to recruit patients across departments and diagnoses by means of the central electronic patient record system. The RCs were able to carry out the interviews with 50% (237/476) of the patients in the hospital setting while 31% were contacted by telephone. The remaining 19% were not interviewed (46 patients were unavailable for practical reasons, 18 died, 11 were demented or mentally disabled, nine were too weak, two had a language barrier, one unexplained and four did not want to participate). The RCs experienced that the setting (often less than optimal in crowded hospital wards) was of relatively little importance, while patients valued the fact that the conversation was dedicated to their specific needs beyond the medical treatment. Among patients interviewed in the hospital wards 30% (72/237) were accompanied by a relative or friend. The RCs experienced that the patients were very interested in participating and satisfied with the focus of the interview. Interviews by phone were found to be an acceptable alternative.
Nine patients (2%) did not want the RC to contact their GP with information about needs for rehabilitation. For all other patients the GPs were sent an e-mail about cancer rehabilitation in general, suggestions for an active role of the GP and specific information about the individual needs of the patient. The RCs contacted the GP by phone concerning 46% (216/471) of the patients in the intervention group (using an average of 1.8 telephone calls). In 20% of the patients it was considered that there was no actual rehabilitation needs to report and the GP therefore only received the written information. In the remaining 32% of the cases, contact to the GP could not be obtained (after an average of 2.3 attempts). Of the 164 practices in the intervention group 117 (71%) were reached by phone at least once, while 47 practices were not. Among the latter, 19 had only patients not interviewed and three had only patients who did not allow information to be passed on to the GP. In other words, 15% (25) of the practices which were contacted in order to inform on needs expressed by one or more patients during interview were unreachable by phone at all attempts.
The RCs experienced that one way to reach the GPs was to arrange with the practice secretary an appointed time to call. The GP's response to the telephone contact were rated by the RCs as “very positive” or “positive” in most cases (92%) and 5% were rated as “neutral” (n=199). Some 3% were rated “critical” or “very critical” of the telephone call (some commented that it was disturbing or had little added value compared to the written information in discharge summaries or a letter from the RC).
Discussion
This study showed that it was feasible to conduct a large-scale RCT to evaluate a complex intervention in the healthcare system. The clinical intervention seemed to be acceptable to both patients and GPs. The patients valued the interview dedicated to needs beyond the medical treatment and accepted information to their GPs. It was possible to reach the GPs by phone for passing on information and the GPs accepted the active information. The degree to which the planned contacts between patients, RCs and GPs were implemented was acceptable.
Initiatives to improve communication and information have previously been tested to overcome the challenges at the interface of primary and secondary care [Citation10,Citation16]. Practical, resource and manpower challenges may hinder implementation of demanding interventions [Citation16]. Within the limited use of health care professionals’ resources we found that all elements of the intervention were, however, feasible. Two nurses worked full-time which may have been crucial for the identification of patients and the feasibility of the clinical work. Accomplishment of the patient interview succeeded in 81% of the cases, which was acceptable. Patient interview by phone has been used in different follow-up programmes [Citation29] and was a feasible alternative to the face-to-face situation. In conclusion, it seems likely that the nurse-led interview targeting rehabilitation needs may work in everyday clinical practice. In a busy ward, however, it will be a challenge to maintain the exclusive focus on rehabilitation during the patient interviews and the personal contact to the GPs.
Nearly all patients accepted that the GP was contacted, which is in line with a previous Danish study [Citation10]. The personal communication to the GPs by phone was given high priority when we designed the intervention. It was often necessary to make several calls, but the experience was that the secretaries generally were helpful to find solutions. Calls were well received by the majority of GPs, who appeared to be interested in a targeted contact about their cancer patients. This is in line with previous reports [Citation30]. In general the GPs and the RCs found the model acceptable and feasible. Communication by phone was possible and may become part of a clinical routine.
This novel approach to cancer rehabilitation requires effect evaluation on patient level. RCTs are widely accepted as the most reliable method of determining effectiveness, and there is an increasing recognition that also nonpharmacological interventions should be rigorously evaluated [Citation31]. Shared care services are often introduced without being piloted or subjected to the rigours of research evaluation [Citation9]. Healthcare systems are known to change over time without any intervention and it is therefore important to use study designs that can handle this problem. The completion of this RCT will add significantly to the sparse evidence of the effect of interventions aiming to facilitate rehabilitation among cancer patients.
Acknowledgements
The authors wish to thank all patients and health care professionals who took part in the study. We also wish to thank Lise Stark, Research Unit of General Practice, University of Southern Denmark, for proofreading the manuscript. This study is funded by the Danish Cancer Society, the Novo Nordisk Foundation and the Region of Southern Denmark. The National Research Center for Cancer Rehabilitation is funded by The Danish Cancer Society. The study has been registered at Clinical-Trials.gov with the registration ID number: NCT01021371.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Mikkelsen TH, Sondergaard J, Jensen AB, Olesen F. Cancer rehabilitation: Psychosocial rehabilitation needs after discharge from hospital? Scand J Prim Health Care 2008;26:216–21.
- Hewitt M, Greenfield S, Stovall E. From cancer patient to cancer survivor: Lost in transition. The National Academies Press, Washington, DC: Comittee on Cancer Survivorship. Improving Care and Quality of Life, National Cancer Policy Board, Institute of Medicine, and National Research Council; 2006.
- Groenvold M, Pedersen C, Jensen CR, Faber MT, Johnsen AT. The cancer patient's world: A study of what Danish cancer patients need – results, assessments and proposals. Research Unit, Palliative Medical Department, H:S/Bispebjerg Hospital, 2006. Danish.
- Schmid-Buchi S, Halfens RJ, Dassen T, van den Borne B. Psychosocial problems and needs of posttreatment patients with breast cancer and their relatives. Eur J Oncol Nurs 2010. Epub Jan 18.
- Ronson A, Body JJ. Psychosocial rehabilitation of cancer patients after curative therapy. Support Care Cancer 2002; 10:281–91.
- Earle CC. Failing to plan is planning to fail: Improving the quality of care with survivorship care plans. J Clin Oncol 2006;24:5112–6.
- Gilbert SM, Miller DC, Hollenbeck BK, Montie JE, Wei JT. Cancer survivorship: Challenges and changing paradigms. J Urol 2008;179:431–8.
- Houlihan NG. Transitioning to cancer survivorship: Plans of care. Oncology (Williston Park) 2009;23(8 Suppl):42–8.
- Smith SM, Allwright S, O'Dowd T. Does sharing care across the primary-specialty interface improve outcomes in chronic disease? A systematic review. Am J Manag Care 2008;14:213–24.
- Nielsen JD, Palshof T, Mainz J, Jensen AB, Olesen F. Randomised controlled trial of a shared care programme for newly referred cancer patients: Bridging the gap between general practice and hospital. Qual Saf Health Care 2003; 12:263–72.
- Grunfeld E. Primary care physicians and oncologists are players on the same team. J Clin Oncol 2008;26: 2246–7.
- Grunfeld E. Cancer survivorship: A challenge for primary care physicians. Br J Gen Pract 2005;55:741–2.
- Johansson B, Berglund G, Hoffman K, Glimelius B, Sjoden PO. The role of the general practitioner in cancer care and the effect of an extended information routine. Scand J Prim Health Care 2000;18:143–8.
- Kendall M, Boyd K, Campbell C, Cormie P, Fife S, Thomas K, . How do people with cancer wish to be cared for in primary care? Serial discussion groups of patients and carers. Fam Pract 2006;23:644–50.
- Anvik T, Holtedahl KA, Mikalsen H. “When patients have cancer, they stop seeing me” – the role of the general practitioner in early follow-up of patients with cancer – a qualitative study. BMC Fam Pract 2006;7:19.
- Grunfeld E, Earle CC. The interface between primary and oncology specialty care: Treatment through survivorship. J Natl Cancer Inst Monogr 2010;2010:25–30.
- Bulsara C, Ward AM, Joske D. Patient perceptions of the GP role in cancer management. Aust Fam Physician 2005; 34:299–300, 302.
- Moher D, Hopewell S, Schulz KF, Montori V, Gotzsche PC, Devereaux PJ, . CONSORT 2010 Explanation and Elaboration: Updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c869.
- Information available at the homepage of the Region of Southern Denmark. Available from: www.regionsyddanmark.dk (In Danish).
- PLO Praksistælling [General Practitioners’ Organisation Practice Count] Copenhagen, 2009. Available from: www.laeger.dk/portal/page/portal/LAEGERDK/Laegerdk/P_L_O/Om%20PLO/Tal%20og%20publikationer/Statistik%20om%20almen%20praksis/praksist%C3%A6lling_2009.pdf (In Danish).
- Mikkelsen T, Sondergaard J, Sokolowski I, Jensen A, Olesen F. Cancer survivors’ rehabilitation needs in a primary health care context. Fam Pract 2009;26:221–30.
- Kurtz SM, Silverman JD. The Calgary-Cambridge Referenced Observation Guides: An aid to defining the curriculum and organizing the teaching in communication training programmes. Med Educ 1996;30:83–9.
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, . The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365–76.
- Groenvold M, Klee MC, Sprangers MA, Aaronson NK. Validation of the EORTC QLQ-C30 quality of life questionnaire through combined qualitative and quantitative assessment of patient-observer agreement. J Clin Epidemiol 1997; 50:441–50.
- Baker F, Denniston M, Zabora J, Polland A, Dudley WN. A POMS short form for cancer patients: Psychometric and structural evaluation. Psychooncology 2002;11:273–81.
- Vedsted P, Sokolowski I, Heje HN. Data quality and confirmatory factor analysis of the Danish EUROPEP questionnaire on patient evaluation of general practice. Scand J Prim Health Care 2008;26:174–80.
- Wallston KA, Wallston BS, DeVellis R. Development of the Multidimensional Health Locus of Control (MHLC) Scales. Health Educ Monogr 1978;6:160–70.
- Peterman AH, Fitchett G, Brady MJ, Hernandez L, Cella D. Measuring spiritual well-being in people with cancer: The functional assessment of chronic illness therapy – Spiritual Well-being Scale (FACIT-Sp). Ann Behav Med 2002;24:49–58.
- Aranda S, Schofield P, Weih L, Milne D, Yates P, Faulkner R. Meeting the support and information needs of women with advanced breast cancer: A randomised controlled trial. Br J Cancer 2006;95:667–73.
- Mikkelsen TH. PhD-thesis: Cancer rehabilitation in Denmark – with particular focus on the present and future role of general practice. Faculty of Health Sciences, University of Aarhus, Denmark, 2009. ISBN 978-87-90004-09-5.
- Campbell M, Fitzpatrick R, Haines A, Kinmonth AL, Sandercock P, Spiegelhalter D, . Framework for design and evaluation of complex interventions to improve health. BMJ 2000;321:694–6.