Abstract
Background. Many patients treated with radiotherapy to the pelvic region report a change in bowel habits. Loose stools, urgency and fecal incontinence may have a significant impact on daily life and social functioning. Material and methods. We attempted to follow up 789 women, treated with pelvic radiotherapy for a gynecological cancer during 1991 to 2003 at two departments of gynecological oncology in Sweden. A control group of 478 women from the Swedish Population Registry was also included. As a preparatory study, we made in-depth interviews with 26 women previously treated for gynecological cancer. Based on their narratives, we constructed a study-specific questionnaire including 351 questions and validated it face-to-face. The questionnaire covered questions of physical symptoms originating in the pelvis, demographics, psychological and quality of life factors. In relation to bowel symptoms, 60 questions were asked. Results. Six-hundred and sixteen (78%) gynecological cancer survivors and 344 (72%) control women participated. Two-hundred and twenty-six (37%) cancer survivors reported loose stools at least once a week. Eighty-three percent of the survivors with loose stools every day reported defecation urgency with fecal leakage, compared to 20% of cancer survivors without loose stools. Cancer survivors with loose stools at least once a week were 7.7 times more likely to suffer from defecation urgency with fecal leakage (95% CI 4.4–13.3) compared to those who had loose stools once a month or less. In order to avoid loose stools affected survivors with loose stools often skipped meals (13%), made an active choice of food (47%) and preferentially used prescribed medication (36%). Discussion. There is a relation between loose stools and defecation urgency with fecal leakage among long-term gynecological cancer survivors treated with pelvic radiotherapy. Targeting loose stools can possibly help survivors to decrease frequency of fecal leakage.
The population of long-term gynecological cancer survivors is growing and many have late gastrointestinal side-effects after radiotherapy. Clinical experience suggests that fecal incontinence, one of the most troubling sources of impaired quality of life and social functioning, may be related to loose stools and defecation urgency. Increasing the understanding of this relation, and other consequences of loose stools, may provide guidance for targeted intervention to those affected.
Reported incidence of fecal incontinence among cancer survivors treated with pelvic radiotherapy varies between 3% and 53% [Citation1–3]. We recently described an occurrence of defecation urgency with accompanying fecal leakage of 49% among long-term gynecological cancer survivors compared to 12% among control women from the general population [Citation4].
Loose stools (“diarrhea”) and defecation urgency are common late symptoms after pelvic radiotherapy with reported incidence rates varying between 14 to 69% [Citation2,Citation5,Citation6] and around 50%, respectively [Citation4,Citation5,Citation7]. If a relationship between loose stools and fecal incontinence exists, it is plausible that preventive measures and interventions against loose stools will decrease the occurrence or alleviate the symptoms of fecal incontinence.
Personal identity numbers and official population-based registers in Sweden, covering all Swedish citizens, offer exceptionally good conditions for studying cancer survivors without selection-induced problems. We performed a population-based study on gynecological cancer survivors concerning long-lasting symptoms after pelvic radiotherapy including physical and psychological symptoms, sexuality, quality of life and social functioning [Citation4]. Here we report on the occurrence of loose stools and its relation to certain other factors, including fecal incontinence.
Material and methods
Study population
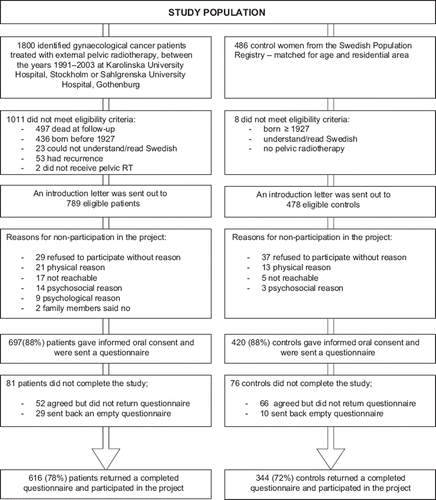
We identified a cohort of 1800 women treated between 1991–2003 with external pelvic radiotherapy for a gynecological malignancy at Radiumhemmet, Karolinska University Hospital in Stockholm, and at Jubileumskliniken, Sahlgrenska University Hospital in Gothenburg of whom 1 303 were alive in 2004. After excluding patients who did not meet the eligibility criteria of being born 1927 or later, could read and understand Swedish and had not experienced a recurrence of their malignancy, 789 patients remained and were invited.
As controls we recruited 486 women from the Swedish Population Register, matched for age and residency and who had not had pelvic radiotherapy. An error in the matching procedure led to a younger control population (mean age 58.0) compared to the cancer survivors (mean age 64.4) which was adjusted for in the analyses. The regional ethics committees approved of the study.
Questionnaire
Our methods for studies of cancer survivorship and development of a study-specific questionnaire have been documented in more than 80 scientific papers [Citation8,Citation9]. A description of the development and validation of the present questionnaire has previously been reported [Citation4]. The questionnaire was developed during a qualitative phase lasting 18 months, starting with interviews of cancer survivors until no new information was identified. In total 26 women shared their experiences. Based on the interviews, a study-specific questionnaire was constructed consisting of 351 questions covering symptoms from the gastrointestinal tract, the bladder, genitals, pelvic bones and the legs. Questions concerning demographics, sexual dysfunction, psychological and quality-of-life issues as well as social functioning were included. In each part of the constructed questionnaire we asked about the incidence, prevalence, intensity and duration of the symptoms when appropriate. For example: “Have you had loose stools during the past six months?” with the possible answers: “No”, “Yes, occasionally”, “Yes, at least once a month”, “Yes, at least once a week”, “Yes, at least three times a week”, “Yes, at least once a day”.
The final version was tested for face validity in 20 individuals. The questionnaire was thereafter tested in a pilot study including yet another 20 gynecological cancer survivors within the study population. We obtained a participation rate of 80% which was regarded as sufficient to continue with the main study. The main study, the quantitative phase, was carried out from January to October 2006. Eligible women who gave informed consent received a postal questionnaire. To maintain anonymity, each questionnaire contained a number for identification.
Medical records were reviewed to confirm the cancer diagnosis, stage of disease and treatment modalities regarding surgery, radiotherapy and chemotherapy. Patients with recurrent disease were excluded. Treatment was according to current local treatment programs and applied study protocols which were ongoing at the time of treatment. These protocols and recommendations have changed over the studied period.
The results from the questionnaire and the data from the medical records were coded and transferred to the freeware data entry and validation program Epi-Data (www.epidata.dk).
Radiotherapy
Three-dimensional conformal treatment planning based on computed tomography scans was used for external beam radiotherapy treatment (EBRT) planning. Patients received EBRT by a linear accelerator delivering between 6 to 50 megavoltage photons. Before 1996 a technique with two opposing fields was predominant, but from 1996 it was more common to use a four-field box technique. It was also more common to use lower daily fractionation, 1.6 to 1.8 Gy per day in the first years of the studied period. Later 1.8 to 2.0 Gy per day became the standard fractionation. The brachytherapy (BT) was based on standardized techniques using applicator templates. The dose was prescribed according to local practice.
For endometrial cancer the EBRT was prescribed as either 38 Gy at 2 Gy per fraction or 39.6 Gy at 1.8 Gy per fraction in Stockholm or 46 Gy at 2 Gy per fraction in Gothenburg. For early stages of cervical cancer the prescribed EBRT dose was 45 Gy at 1.6 Gy daily in Stockholm. Patients treated with preoperative brachytherapy received EBRT with central shielding. Until 2001 prophylactic paraaortic treatment to 40 Gy at 1.6 Gy daily was given in Stockholm to patients with pelvic lymph node metastasis. In Gothenburg the prescribed EBRT dose to the pelvic region was 46 Gy at 2 Gy per fraction during the whole study period. In 2001 treatment with postoperative concomitant chemoradiotherapy with weekly cisplatinum (40 mg/m2) was introduced into clinical practice in Stockholm. For locally advanced stages of cervical cancer the prescribed total EBRT dose in Stockholm was 50 Gy in patients having BT and 60 Gy without BT. In Gothenburg the prescribed total EBRT dose was 55 Gy in patients having BT and 67 Gy in patients without BT. Neoadjuvant platinum-based combination of chemotherapy was given prior to EBRT in Gothenburg. In Stockholm concomitant chemoradiotherapy with weekly cisplatinum was gradually introduced starting in 1999 and continuing thereafter.
The prescribed total dose for ovarian- and fallopian tube cancer was 20 Gy to the whole abdomen with an additional 20 Gy to a lower abdominal field, for uterine sarcoma 50.4 Gy to the pelvic region, delivered in daily fractions of 1.8 Gy in the same way as for the endometrial cancer. The prescribed radiotherapy for vaginal cancer was similar to cervical cancer patients.
Surgery
Surgery, consisting of hysterectomy and bilateral salpingoophorectomy, with or without omentectomy, was performed on all patients having endometrial and ovarian cancers, cancer of the fallopian tube and uterine sarcoma. Pelvic and (or) paraaortic lymphadenectomy was rarely performed. Surgery for cervical cancer typically consisted of radical hysterectomy with bilateral salpingo-oophorectomy and pelvic lymphadenectomy. Vaginal cancer was treated with a procedure similar to that used for cervical cancer but with less extensive surgery.
Statistical analysis
The symptom “loose stools” was dichotomized into having the symptom “at least once a week or more often” or not. The reason for choosing this cut-off level was due to the high prevalence of the symptom among control women (69% reported loose stools occasionally or more often), while only 14% reported the symptom at least once a week. In we compared for certain factors across categories, the percentages of survivors having loose stools with the percentage of survivors without or with very rare loose stools (). We cite the percentage ratios as the measure of the association. All calculations were performed using the SAS statistical software package (version 9).
Table I. Characteristics of gynecological cancer survivors and control women.
Results
Among the cancer survivors 616 (78%) returned a completed questionnaire, corresponding figures for populations controls were 344 (72%) (). Eight women, who reported having a stoma, were excluded from the analysis. Two-hundred and thirty-four (39%) cancer survivors stated that they had loose stools at least once a week, whereas the prevalence of loose stools at least once a week among control women was 48 (14%) giving a percentage ratio of 2.7 (95% CI 2.3–4.1).
Demographics and clinical characteristics are presented in . Thirty-one percent of the cancer survivors with loose stools at least once a week were current smokers compared to 19% among survivors with loose stools being experienced less frequently. Having experienced episiotomy and vacuum delivery when giving birth was more common among survivors who frequently experienced loose stools. These women also more frequently reported anal-sphincter injury, abdominal surgery, lactose intolerance and treatment for bowel-related co morbidities from the bowel ().
Of those women who experienced loose stools at least once a week, 54% had endometrial cancer compared to 63% among those who had loose stools monthly or less (). Forty percent of the cancer survivors with loose stools at least once a week were treated between 30 to 60 months prior to follow-up in 2006, compared to 30% among survivors who experienced loose stools monthly or less often.
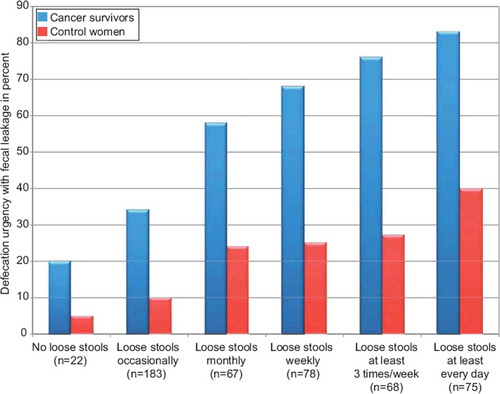
The prevalence of loose stools was related to the occurrence of defecation urgency with fecal leakage. Eighty-three percent (62 of 75) of the survivors with loose stools every day reported having the symptom defecation urgency with fecal leakage, compared to 20% of cancer survivors without loose stools (). The corresponding figures for control women were 40% and 5%, respectively.
Figure 2. Relation between loose stools and defecation urgency with fecal leakage among gynecological cancer survivors and control women. Absolute numbers of survivors with loose stools are presented within parenthesis.
Cancer survivors with loose stools at least once a week experienced more defecation urgency with fecal leakage (percentage ratio 7.7), and more emptying of all stools into clothing without forewarning (percentage ratio 13.0), at least once a month, compared to survivors who experienced loose stools monthly or less often (). Having loose stools at least once a week was associated with leakage of loose stools at least while awake (percentage ratio 18.5). Few survivors had leakage of loose stools while asleep ().
Table II. Loose stools and fecal incontinence symptoms among gynecological cancer survivors.
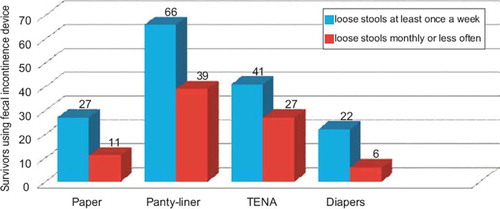
One fifth of the survivors with loose stools at least once a week reported increased problems with loose stools when stressed, occurring at least once a week with a percentage ratio of 35.8. Having loose stools at least once a week was associated with skipping meals (percentage ratio 24.3), an active choice of food and used prescribed medication (percentage ratio 4.2) to avoid loose stools (). Sixty-nine percent of affected survivors used some kind of protection device, 50% panty-liners and 50% incontinence pads “TENA” or diapers ().
Table III. Strategies used by gynecological cancer survivors to avoid loose stools.
Discussion
We found an association between loose stools and defecation urgency with fecal leakage among long-term gynecological cancer survivors treated with pelvic radiotherapy alone or as part of combined treatment. To avoid loose stools affected cancer survivors skipped meals and made an active choice of what to eat, at least once a week.
Long-lasting gastrointestinal side-effects after radiation, in particular loose stools and defecation urgency, are common [Citation2,Citation5,Citation6] and may contribute to aggravate fecal incontinence. Our population-based observation of a strong relationship between the frequency of loose stools and the occurrence of defecation urgency with fecal leakage after radiotherapy has to our knowledge not previously been demonstrated. In the PORTEC-2 study 348 cancer survivors, treated with either vaginal brachytherapy or external radiotherapy for endometrial cancer, reported symptoms on EORTC QLQ-C-30 [Citation10] questionnaire. Survivors treated with external radiotherapy reported higher mean score of diarrhea and fecal incontinence at a two year follow-up compared to those treated with brachytherapy; the authors do not report the correlation between the symptoms [Citation6].
A number of possible mechanisms leading to long-lasting loose stools after pelvic radiotherapy have been reported including changes in small bowel motility predisposing to bacterial overgrowth and occasionally pseudo-obstruction, bile salt malabsorption, lactose malabsorption and increased small and large-bowel transit time [Citation11,Citation12]. Other factors may also contribute to the development of loose stools. Andreyev and co-workers investigated 265 consecutive patients referred to their gastroenterology clinic, during a 32-month period, for gastrointestinal symptoms starting after radiotherapy [Citation13]. The majority of patients had more than one gastrointestinal diagnosis and one-third of all diagnoses were judged to be unrelated to their previous radiotherapy [Citation13]. These observations underscore the need for proper diagnostic set up by referral of cancer survivors with gastrointestinal symptoms after pelvic radiotherapy for gastroenterological evaluation.
A correlation between smoking and severe side-effects of pelvic radiotherapy for cervical cancer has been reported. In a retrospective study including 3489 patients treated with radiotherapy for stage I or II cervical cancer, Eifel and co-workers report a strong correlation (hazard ratio 3.25; 95% CI 2.21–4.78) between smoking and major small bowel complications (defined as symptoms occurring or persisting more than three months after treatment and that required hospitalization, blood transfusion, surgery or leading to death) [Citation14]. In our study, current smokers were more prevalent among the survivors with frequent loose stools. We also noted an increased occurrence of loose stools in relation to stress, previously described in relation to patients with inflammatory bowel diseases [Citation15]. Only one survivor had ulcerative colitis, another Morbus Crohn and additional 13 Inflammatory Bowel Syndrome (IBS). We adjusted for risk factors for loose stools, smoking and IBS, with by and large unchanged results (data not shown).
Among long-term cancer survivors with late appearing fecal incontinence following radiotherapy, the picture is even more complex due to the multifaceted nature of the condition and the interindividual variation. Reasonable anal-sphincter dysfunction due to radiation-induced fibrosis in the sphincter musculature entails one explanation for the increased occurrence of fecal incontinence among cancer survivors; the dose of ionizing radiation to the anal sphincter relates to the occurrence of fecal leakage [Citation16]. But, certainly other factors are involved in the pathophysiology resulting from radiation-induced injury of the anorectal structures [Citation17]. Putta and Andreyev reviewed the literature between 1966 to 2005; they highlight the need for research to determine why disabling symptoms occur in some individuals after radiation and not in others [Citation18]. We do not have enough evidence to suggest thresholds for the mean dose to the anal-sphincter region; we can better define the region by anatomical-radiological means and have a lot to learn about the inherited sensitivity to radiation of these normal-tissue structures [Citation19].
Several other conditions are associated with an increased risk of fecal incontinence. Among background population the reported risk factors for fecal incontinence includes increasing age, obesity, neurological disorders, perineal-, anal and vaginal injuries, fetal weight, rectal diseases and chronic gastrointestinal conditions such as constipation and diarrhea [Citation20]. The contribution of bowel disturbances to fecal incontinence has further been investigated. In a study comprising 507 women with fecal incontinence in the 12 preceding months and 127 randomly selected controls Bharucha and co-workers compared bowel habits between the two groups using diaries to assess bowel habits. They reported that rectal urgency, a sense of incomplete evacuation, stool frequency, and form were independent risk factors for fecal incontinence among women in the community [Citation21]. These results are consistent with the results among control women in our study for whom the age-adjusted percentage ratio for defecation urgency with fecal leakage was 3.6 (95% CI; 1.5–9.0) comparing control women with and without loose stools (data not shown).
The cancer survivors in our study tried different remedies to avoid loose stools including prescribed and over-the-counter medication as well as complementary therapies. Other strategies used by the cancer survivors were to make an active choice what to eat and skipping meals. Abayomi and co-workers asked 117 women, who had completed radiotherapy for cervical or endometrial cancer, to complete a questionnaire exploring bowel problems, coping measures and quality of life. They found that a considerable number of women “took care with how much food they ate” to avoid gastrointestinal symptoms [Citation22]. This is also confirmed by Gillispie and co-workers who investigated day-to-day impact of gastrointestinal symptoms in patients after pelvic radiotherapy [Citation23]. The most common intervention in this study was a change of diet, followed by prescribed medications predominantly anti-diarrheal medications [Citation23]. We have no clear data concerning the efficacy of dietary manipulations to alleviate bowel symptom after pelvic radiotherapy [Citation24].
Among cancer survivors in the current study with loose stools at least once a week the occurrence of defecation urgency with fecal leakage was over 60%. In contrast, only 36% reported the use of prescribed medication for the treatment of loose stools. Data implies that less than 20% of cancer survivors with gastrointestinal symptoms are referred to a gastroenterology specialist [Citation25] and we presume that in the absence of seeking medical professionals the cancer survivors implement their own strategies in order to manage symptoms.
Adjuvant external pelvic radiotherapy has until recently been widely used in Sweden and globally. Recently, several randomized trials in intermediate risk endometrial cancer have shown a risk reduction of vaginal recurrence but without a survival benefit which has led to a decrease in the use of adjuvant therapy for stage 1 endometrial cancer [Citation26–29]. The results of a prospective Swedish-Norwegian study suggest that whole abdominal radiotherapy may be an option as consolidation treatment for selected subgroups of patients with ovarian cancer [Citation30] but is currently rarely used.
We work with epidemiological methods as adapted in this field by the hierarchical step model for study design and data interpretation [Citation31]. Strengths of this study include the population-based setting, and the reasonably high participation rate. Application of multiple validated measurements, comprehensive face-to-face validation of outcomes and the use of a questionnaire answered in private, lowered the risk for measurement errors and eliminated interviewer-induced bias. We cannot exclude the possibility that non-participating subjects would have answered differently. Surgery might result in damage to some of the autonomic nerves to the bowel disturbing initiation of defecation or emptying reflex. Based on our results, we cannot judge to what degree radiotherapy, surgery, chemotherapy or any other factor induces the increased occurrence among cancer survivors. Women more than 80 years old did not participate and we do not know whether we generalize our findings concerning a relation between loose stools and fecal incontinence to this category of elderly women, nor to other cultural setting than ours with, for example, another dietary pattern. The proportion of women with loose stools at least once a week treated within less than five years was 10% higher compared to the women treated between five and ten years and more, respectively. In this cross-sectional approach we cannot disentangle the causal factors or the influence of time, however, our main interest was the association between loose stools and fecal incontinence.
Diagnostic procedures and therapies targeting loose stools may alleviate distressing fecal incontinence after pelvic radiotherapy. We suggest that health care providers actively ask patients about gastrointestinal symptoms, including fecal incontinence, after treatment and refer affected patients to specialists in gastroenterology in order to avoid trial- and error management. We believe it is necessary to develop greater disciplinary cooperation to help affected women. The role of smoking cessation among cancer survivors deserves further attention: smoking cessation may be fruitful to diminish the frequency or intensity of loose stools and fecal incontinence among smoking cancer survivors.
Acknowledgements
This study was funded by grants from the Swedish Cancer Society, The Cancer Research Funds of Radiumhemmet, The King Gustav V Jubilee Clinic Cancer Foundation, Gothenburg and The Swedish State under the ALF agreement, Gothenburg. We thank the gynecological cancer-survivors and all other women who participated in this study.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- al-Abany M, Helgason AR, Cronqvist AK, Svensson C, Wersall P, Steineck G. Long-term symptoms after external beam radiation therapy for prostate cancer with three or four fields. Acta Oncol 2002;41:532–42.
- Gami B, Harrington K, Blake P, Dearnaley D, Tait D, Davies J, . How patients manage gastrointestinal symptoms after pelvic radiotherapy. Aliment Pharmacol Ther 2003;18:987–94.
- Yeoh E, Sun WM, Russo A, Ibanez L, Horowitz M. A retrospective study of the effects of pelvic irradiation for gynecological cancer on anorectal function. Int J Radiat Oncol Biol Phys 1996;35:1003–10.
- Dunberger G, Lind H, Steineck G, Waldenstrom AC, Nyberg T, Al-Abany M, . Self-reported symptoms of faecal incontinence among long-term gynaecological cancer survivors and population-based controls. Eur J Cancer 2010;46: 606–15.
- Haddock MG, Sloan JA, Bollinger JW, Soori G, Steen PD, Martenson JA. Patient assessment of bowel function during and after pelvic radiotherapy: Results of a prospective phase III North Central Cancer Treatment Group clinical trial. J Clin Oncol 2007;25:1255–9.
- Nout RA, Putter H, Jurgenliemk-Schulz IM, Jobsen JJ, Lutgens LC, van der Steen-Banasik EM, . Quality of life after pelvic radiotherapy or vaginal brachytherapy for endometrial cancer: First results of the randomized PORTEC-2 trial. J Clin Oncol 2009;27:3547–56.
- Creutzberg CL, van Putten WL, Koper PC, Lybeert ML, Jobsen JJ, Warlam-Rodenhuis CC, . Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: Multicentre randomised trial. PORTEC Study Group. Post Operative Radiation Therapy in Endometrial Carcinoma. Lancet 2000;355(9213): 1404–11.
- Bergmark K, Avall-Lundqvist E, Dickman PW, Henningsohn L, Steineck G. Vaginal changes and sexuality in women with a history of cervical cancer. N Engl J Med 1999;340: 1383–9.
- Steineck G, Helgesen F, Adolfsson J, Dickman PW, Johansson JE, Norlen BJ, . Quality of life after radical prostatectomy or watchful waiting. N Engl J Med 2002;347: 790–6.
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, . The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365–76.
- Andreyev J. Gastrointestinal symptoms after pelvic radiotherapy: A new understanding to improve management of symptomatic patients. Lancet Oncol 2007;8:1007–17.
- Danielsson A, Nyhlin H, Persson H, Stendahl U, Stenling R, Suhr O. Chronic diarrhoea after radiotherapy for gynaecological cancer: Occurrence and aetiology. Gut 1991;32:1180–7.
- Andreyev HJ, Vlavianos P, Blake P, Dearnaley D, Norman AR, Tait D. Gastrointestinal symptoms after pelvic radiotherapy: Role for the gastroenterologist? Int J Radiat Oncol Biol Phys 2005;62:1464–71.
- Eifel PJ, Jhingran A, Bodurka DC, Levenback C, Thames H. Correlation of smoking history and other patient characteristics with major complications of pelvic radiation therapy for cervical cancer. J Clin Oncol 2002;20:3651–7.
- Rampton D. Does stress influence inflammatory bowel disease? The clinical data. Dig Dis 2009;27(Suppl 1):76–9.
- al-Abany M, Helgason AR, Cronqvist AK, Lind B, Mavroidis P, Wersall P, . Toward a definition of a threshold for harmless doses to the anal-sphincter region and the rectum. Int J Radiat Oncol Biol Phys 2005;61:1035–44.
- Petersen S, Jongen J, Petersen C, Sailer M. Radiation-induced sequelae affecting the continence organ: Incidence, pathogenesis, and treatment. Dis Colon Rectum 2007;50:1466–74.
- Putta S, Andreyev HJ. Faecal incontinence: A late side-effect of pelvic radiotherapy. Clin Oncol (R Coll Radiol) 2005;17: 469–77.
- Ho AY, Atencio DP, Peters S, Stock RG, Formenti SC, Cesaretti JA, . Genetic predictors of adverse radiotherapy effects: The Gene-PARE project. Int J Radiat Oncol Biol Phys 2006;65:646–55.
- Landefeld CS, Bowers BJ, Feld AD, Hartmann KE, Hoffman E, Ingber MJ, . National Institutes of Health state-of-the-science conference statement: Prevention of fecal and urinary incontinence in adults. Ann Intern Med 2008;148: 449–58.
- Bharucha AE, Seide BM, Zinsmeister AR, Melton LJ, 3rd. Relation of bowel habits to fecal incontinence in women. Am J Gastroenterol 2008;103:1470–5.
- Abayomi J, Kirwan J, Hackett A. The prevalence of chronic radiation enteritis following radiotherapy for cervical or endometrial cancer and its impact on quality of life. Eur J Oncol Nurs 2009;13:262–7.
- Gillespie C, Goode C, Hackett C, Andreyev HJ. The clinical needs of patients with chronic gastrointestinal symptoms after pelvic radiotherapy. Aliment Pharmacol Ther 2007;26: 555–63.
- Sekhon S. Chronic radiation enteritis: Women's food tolerances after radiation treatment for gynecologic cancer. J Am Diet Assoc 2000;100:941–3.
- Andreyev J. Gastrointestinal complications of pelvic radiotherapy: Are they of any importance? Gut 2005;54:1051–4.
- Keys HM, Roberts JA, Brunetto VL, Zaino RJ, Spirtos NM, Bloss JD, . A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: A Gynecologic Oncology Group study. Gynecol Oncol 2004;92:744–51.
- Nout RA, Smit VT, Putter H, Jurgenliemk-Schulz IM, Jobsen JJ, Lutgens LC, . Vaginal brachytherapy versus pelvic external beam radiotherapy for patients with endometrial cancer of high-intermediate risk (PORTEC-2): An open-label, non-inferiority, randomised trial. Lancet 2010;375(9717):816–23.
- Blake P, Swart AM, Orton J, Kitchener H, Whelan T, Lukka H, . Adjuvant external beam radiotherapy in the treatment of endometrial cancer (MRC ASTEC and NCIC CTG EN.5 randomised trials): Pooled trial results, systematic review, and meta-analysis. Lancet 2009;373(9658):137–46.
- Creutzberg CL. GOG-99: Ending the controversy regarding pelvic radiotherapy for endometrial carcinoma? Gynecol Oncol 2004;92:740–3.
- Sorbe B. Consolidation treatment of advanced ovarian carcinoma with radiotherapy after induction chemotherapy. Int J Gynecol Cancer 2003;13(Suppl 2):192–5.
- Steineck G, Hunt H, Adolfsson J. A hierarchical step-model for causation of bias-evaluating cancer treatment with epidemiological methods. Acta Oncol 2006;45:421–9.