Abstract
Background. Gold(III) meso-tetraphenylporphyrin (gold-1a) has previously been shown to prolong the survival of hepatocellular carcinoma (HCC)-bearing rats and nasopharyngeal carcinoma (NPC) metastasis-bearing mice. It has also been proved to inhibit the tumor growth of mice bearing NPC, neuroblastoma and colon carcinoma. Mechanistically, gold-1a induces apoptosis, inhibits cell migration and invasion. In this study the efficacies of gold-1a in inhibiting melanoma and angiogenesis were investigated. Material and methods. A mouse melanoma model was used to investigate the efficacy of gold-1a in inhibiting angiogenesis, tumor growth and prolonging the survival of the tumor-bearing animals. The model was established by inoculation of 2 × 105 B16-F1 mouse melanoma cells into the right back flanks of the mice by subcutaneous inoculation. When the tumors grew to 0.2–0.4 cm in diameters, the mice were treated with gold-1a, solvent control or dacarbazine (DTIC) for comparison. Tumor sizes and animal survivals were monitored throughout the experiment. Tumor tissues were collected and immunohistochemically stained with CD31 antibodies for evaluation of intra-tumoral microvessel density (iMVD). Results and conclusion. Gold-1a significantly prolonged the survivals, reduced angiogenesis and tumor growth rates of melanoma-bearing mice. The compound induced necrosis and apoptosis in the mouse melanoma tissues. Gold-1a also downregulated the expression of genes playing roles in angiogenesis. Gold-1a may potentially be used to treat melanoma in patients.
Melanoma, the most malignant type of skin cancer, is tumor of the melanocytes. The estimated new cases and deaths of melanoma in the United States are 68 130 and 8700 respectively in 2010 [Citation1]. Surgical resection remains the primary treatment for early-stage melanoma. For patients at more advanced stages, adjuvant therapy is needed. DTIC was the first drug approved by Food and Drug Administration for the treatment of advanced-stage melanoma. The overall response rate of DTIC in melanoma patients was 22% but the drug failed to improve survival [Citation2]. Other adjuvants against melanoma in use to date include interleukin-2 (IL-2) and interferon alpha-2b (IFNα-2b). In a phase III randomized trial, the overall response rate of patients to a combination of three chemotherapeutic agents: cisplatin, vinblastine and DTIC (CVD) was 25%, whereas the overall response rate of patients to a combination of CVD plus IL-2 and IFNα-2b was 48%. However, in both cases, there was no improvement in survival. To date, there is no effective therapeutic regimen for melanoma management. As a result, it is urgent to develop new agents for the treatment of advanced melanoma.
Gold compounds have long been shown to own promising medical values [Citation3,Citation4], notable examples are the anti-arthritic activities of gold(I) phosphine compound auranofin and its derivatives [Citation5]. Their anti-cancer properties were exposed since Lorber and co-workers had studied the anti-proliferative activity of auranofin in HeLa cells in 1979 [Citation6,Citation7]. Moreover, gold(III) ion is isoelectronic with platinum(II) ion and forms similar square-planar complexes as in cisplatin, which has been used in treating a variety of cancers. This fact instigated the investigations on the use of gold compounds in cancer treatment. However, due to the tendency of gold(III) metal center to reduce to gold(I) and metallic gold under physiological conditions, only a few of them exhibited satisfactory stability. By using various kinds of dianionic tetradentate ligands, we have synthesized a series of gold(III) complexes which are stable against demetallation under physiological conditions [Citation8,Citation9]. Among them, gold-1a was effective in prolonging the survival of HCC-bearing rats [Citation10] and NPC metastasis-bearing mice [Citation11]. The compound also inhibited tumor growth in mice bearing NPC [Citation12], neuroblastoma [Citation13] and colon carcinoma [Citation14]. Mechanistically, gold-1a has been shown to induce apoptosis [Citation10,Citation12,Citation14,Citation15]; inhibit tumor cell migration and invasion [Citation11].
The anti-cancer properties of gold(III) complexes have been known for three decades [Citation6,Citation7]. Nevertheless, their anti-angiogenic properties are completely unknown. Angiogenesis is the formation of new blood vessels from the pre-existing ones. It plays a pivotal role in tumor growth and metastasis [Citation16], as tumor cells need oxygen and nutrient supply from blood vessels [Citation17]. Inhibition of angiogenesis is thus one of the most important approaches in anti-cancer treatment.
In the present study, we employed a B16-F1 melanoma model to examine the anti-cancer activity of gold-1a as compared to DTIC in treating melanoma in vivo. The in vivo and in vitro anti-angiogenic properties of gold-1a were also examined. Moreover, genes potentially playing roles in gold-1a-mediated anti-angiogenic activities were also studied.
Materials and methods
Cell lines, growth media, growth conditions and drugs
MS1 mouse pancreatic islet endothelial cell line and B16-F1 mouse melanoma cell line were purchased from the American Type Culture Collection (Manassas, VA). Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS) and trypsin-EDTA were products of Invitrogen (Carlsbad, CA). The MS1 and B16-F1 cells were maintained as monolayer culture in DMEM supplemented with 5% FBS and DMEM supplemented with 10% FBS respectively at 37°C, 5% CO2. Gold-1a was synthesized as reported previously [Citation18]. DTIC was purchased from Sigma-Aldrich (St. Louis, MO). Gold-1a and DTIC were reconstituted in dimethyl sulfoxide (DMSO). The reconstituted compounds were further diluted with phosphate-buffered saline (PBS) before injecting into the mice. Cisplatin was purchased from Sigma-Aldrich and was dissolved in DMSO.
Animal model of melanoma and experimental conditions
Female C57BL/6 mice, 6–9 weeks old, weighing 18–22 g, were purchased from the Charles River Laboratories (Wilmington, MA) and cared for according to the guidelines of the Laboratory Animal Unit of the University of Hong Kong (HKU). All animal experiments were conducted under the guidelines approved by the Committee on the Use of Live Animals in Teaching and Research of HKU. Tumors were established by inoculation of 2 × 105 B16-F1 cells suspended in 100 μl PBS into the right back flanks of the mice by subcutaneous injection. When the tumors grew to about 0.2–0.4 cm in diameters, the mice were randomly divided into the following five groups with five mice per group for the survival study and another three mice per group for tissue collection: (1) solvent control (DMSO diluted in PBS); (2) DTIC (40 mg/kg); (3) DTIC (80 mg/kg); (4) gold-1a (0.125 mg/kg) and (5) gold-1a (0.25 mg/kg). The mice were administered with solvent control, DTIC or gold-1a by intraperitoneal injection for ten consecutive days according to the body weight stated in the respective groups, combined with intratumoral injection for once on the first day of treatment according to the body weight (20 mg/kg for both groups treated with DTIC and 0.1 mg/kg for both groups treated with gold-1a). The final concentration of DMSO was less than 2% in PBS when administered to the mice. For the survival study, the mice were either allowed to die naturally or sacrificed when the tumor burden was greater than 10% of the body weight. In both cases the numbers of days they had survived after tumor inoculation were recorded.
Pathological and histological analysis
Tumor volume and body weight were monitored once every three days until the 20th day after tumor inoculation. Tumor volume (V) was calculated by the formula V = ab2 × 0.52, where a and b were the longest and the shortest diameters of the xenografted tumor. Mice used for tissue collection were sacrificed 20 days after tumor inoculation. Xenografted tumors from the mice were excised, fixed in 4% paraformaldehyde for 16 hours, and then embedded in paraffin for histological studies. Paraffin-embedded tissues were sectioned into slices of 5 μm thick for histological studies by hematoxylin and eosin (H & E). The tissue sections were monitored under an inverted microscope at 200× magnification. For the detection of apoptotic cells in the tumor tissues, in situ Cell Death Detection Kit (Roche, Penzberg, Germany) was used and the procedure was described previously [Citation10]. The tissue sections were monitored under an inverted microscope at 320× magnification. The number of apoptotic cells per microscopic field was counted. Intra-tumoral microvessel density (iMVD) was determined by immunohistochemical staining for CD31. Antibody against CD31 was purchased from Novus Biologicals (Littleton, CO). Dewaxed and rehydrated tissue sections were gone through antigen retrieval processes. After blocking, the sections were incubated with anti-CD31 antibody for 1 hour. After washing with PBS, they were further incubated with horse radish peroxidase-conjugated secondary antibody for 30 minutes. The sections were counterstained with haematoxylin, mounted under glass coverslips and observed under an inverted microscope at 100× magnification. Ten fields were randomly chosen and the numbers of microvessels were counted. The number of microvessels per microscopic field was taken as the value of iMVD. The data were shown as mean ± standard error of the mean (SEM) from three mice in each group.
In vitro angiogenesis assay
Endothelial cell growth supplement (ECGS) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). In vitro Angiogenesis Assay Kit was a product of Millipore (Billerica, MA). MS1 mouse pancreatic islet endothelial cell line was employed as a model in the in vitro angiogenesis assay. ECMatrix™ Solution and 10× Diluent Buffer (both provided in the kit) were mixed in a ratio of 9:1 and 50 μl of the mixture was transferred to each well of a 96-well plate. The matrix solution was incubated at 37°C for one hour to allow polymerization to occur. Eighty thousand MS1 cells were then premixed with gold-1a or cisplatin in 100 μl of DMEM supplemented with 5% FBS and 0.05 mg/ml ECGS, and were added to the top of the polymerized matrix. The concentration of DMSO was identical in all wells and its final concentration was ≤ 0.2%. The cells were then incubated at 37°C, 5% CO2 for two hours. At the end of the incubation, 10 μl of 5 mg/ml methylthiazolyldi-tetrazolium bromide (MTT) (USB Corporation, Cleveland, OH) solution was added to each well and the incubation was allowed to continue for another hour. Tube formation was then monitored under an inverted light microscope at 100× magnification.
Cell viability assay
MS1 cells were seeded at a density of 2 × 104 cells per well in 100 μl of medium in a 96-well plate 24 hours before gold-1a treatment. The cells were treated with gold-1a or cisplatin for the same time (two hours) and at the same concentrations as those applied in the in vitro angiogenesis assay. The concentration of DMSO was identical in all wells and its final concentration was ≤ 0.2%. At the end of the incubation period, 10 μl of 5 mg/ml MTT solution was added to each well, and the cells were incubated at 37°C for two hours. Two hundred microliters of acidic isopropanol (0.04 M hydrochloric acid in isopropanol) was then added to each well to dissolve the formazan complexes. Finally, the absorbance of the converted dye was measured at a wavelength of 570 nm with background subtraction at 620 nm. Each condition was done in triplicate and the data were shown as mean ± SEM from three independent experiments.
Quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR)
MS1 cells were seeded to 80% confluent in 10 ml DMEM medium with 5% FBS in a 10-cm dish 24 hours before gold-1a treatment. Cells were untreated or treated with 2 μM gold-1a for 24 or 48 hours. The concentration of DMSO was identical in all dishes (0.02%). Total RNA was then extracted using RNeasy® Mini Kit (Qiagen, Valencia, CA). Oligos were purchased from Integrated DNA Technologies (Coralville, IA). The expression profiles of glyceraldehyde-3-phosphate dehydrogenase (Gapdh) were used to normalize those of the target genes. Forward and reverse primers for mouse Gapdh were 5′ TGGTGCTGCCAAGGCTGTGG 3′ and 5′ TCTCCAGGCGGCACGTCAGA 3′ respectively. Forward and reverse primers for mouse stanniocalcin 1 (Stc1) were 5′ GCAGCATCGCCAAGCGCAAC 3′ and 5′ TGGCCATGTTGGGCCCGATCT 3′ respectively. Forward and reverse primers for mouse vascular endothelial growth factor receptor 2 (Vegfr2) were 5′ ACGGACCGTTAAGCGGGCCA 3′ and 5′ GGCAAGCGTTCACAGCGCTC 3′ respectively. Two hundred nanograms of each total RNA sample was reverse transcribed to cDNA using SuperScript™ II Reverse Transcriptase (Invitrogen). The resulting first strand cDNA was diluted 5-fold and 1 μl of the diluted cDNA was then subjected to quantitative polymerase chain reaction (qPCR), using SYBR® GreenER™ qPCR SuperMix for ABI PRISM® (Invitrogen). The data were shown as mean ± SEM from five independent experiments.
Statistical analysis
Animal survival was analyzed by log-rank test using the GraphPad Prism software (GraphPad Software Inc., San Diego, CA). Number of apoptotic cells, iMVD and relative expression levels were compared by two-tailed Student's t-test (MS Excel). P < 0.05 was considered statistically significant.
Results
Gold-1a prolonged the survival of B16-F1 melanoma-bearing mice
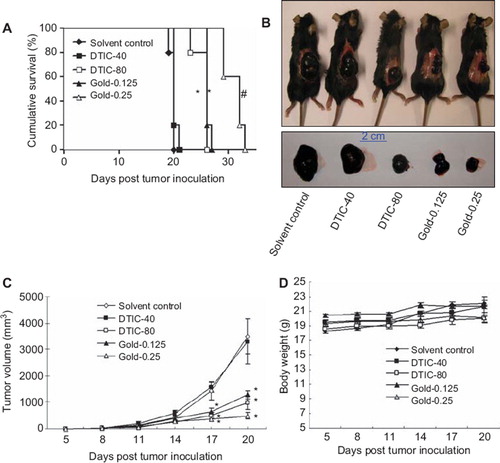
To investigate the in vivo anti-cancer efficacy of gold-1a, a B16-F1 mouse melanoma model was employed. The anti-cancer efficacy of gold-1a was compared to DTIC, the most widely used chemotherapeutic drug for treating melanoma [Citation19]. Our results indicated that the median survival rates of mice in the (1) solvent control; (2) DTIC (40 mg/kg); (3) DTIC (80 mg/kg); (4) gold-1a (0.125 mg/kg) and (5) gold-1a (0.25 mg/kg) groups were 20 days, 20 days, 26 days, 26 days and 32 days after tumor inoculation respectively (). Gold-1a at both dosages significantly prolonged the survival of the melanoma-bearing mice compared to solvent control (p < 0.05), whereas DTIC was effective only at a relatively high dose (80 mg/kg) (p < 0.05). The result indicated that gold-1a was more effective than DTIC, the most widely used chemotherapeutic drug in melanoma, in prolongation of survival of B16-F1 melanoma-bearing mice.
Figure 1. (A) Survival curves of melanoma-bearing mice in different treatment groups. The median survival times of mice in the (1) solvent control; (2) DTIC (40 mg/kg) (DTIC-40); (3) DTIC (80 mg/kg) (DTIC-80); (4) gold-1a (0.125 mg/kg) (Gold-0.125) and (5) gold-1a (0.25 mg/kg) (Gold-0.25) groups were 20 days, 20 days, 26 days, 26 days and 32 days after tumor inoculation respectively. *p < 0.05, compared to solvent control and DTIC-40 groups. #p < 0.05, compared to all other four groups. (B) Morphologies of melanoma-bearing mice and sizes of the tumors in different experimental groups. (C) A plot of tumor volume versus the number of days post tumor inoculation of melanoma-bearing mice in different groups. *p < 0.05, compared to solvent control and DTIC-40 groups. Data were shown as mean ± SEM from five mice in each group. (D) A plot of body weight versus the number of days post tumor inoculation of mice in different groups. There was no significant difference in the body weights of mice in different groups. Data were shown as mean ± SEM from five mice in each group.
Gold-1a inhibited melanoma growth in mice
To investigate the efficacy of gold-1a in inhibiting melanoma growth, tumor sizes of melanoma-bearing mice were measured once every three days throughout the experiment. Tumors of mice in the solvent control and the DTIC (40 mg/kg) groups reached the size of over 3000 mm3 20 days after tumor inoculation ( and ). In contrast, tumors of mice in the DTIC- (80 mg/kg) and gold-1a- (both dosages) treated groups were only slightly over 1000 mm3 at the same time point, and it is noteworthy that the difference in the tumor volumes in these three groups were statistically significant compared to mice in the solvent control and the DTIC (40 mg/kg) groups ( and ). The data indicated that gold-1a inhibited melanoma growth and its effect was superior to that of DTIC. In addition, there was no obvious difference in body weights between mice in different groups (), implying that both DTIC and gold-1a, when applied at the dosages used in the current study, had no adverse effect to the growth of the mice.
Gold-1a induced necrosis and apoptosis in the tumor tissues of B16-F1 melanoma-bearing mice
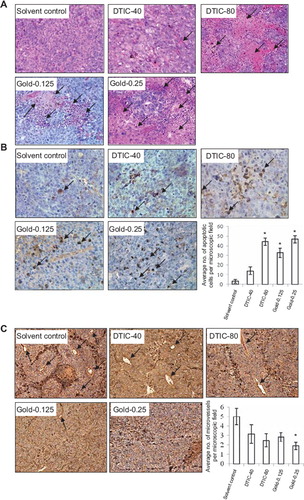
We investigated whether gold-1a induced necrosis and apoptosis in vivo by H & E staining and in situ labeling of apoptosis-induced DNA strand breaks respectively. H & E staining showed that mice treated with gold-1a at 0.125 mg/kg or 0.25 mg/kg contained larger areas of necrosis in the tumor tissues when compared to those treated with solvent control (). In contrast, DTIC induced obvious necrosis only at a relatively high dose (80 mg/kg). At a lower dosage of 40 mg/kg, only a few small necrotic areas could be seen in the tumor tissues. In addition, greater numbers of apoptotic cells were found in the tumor tissues of mice in the gold-1a treated groups (both the higher and the lower doses) compared to the solvent control group (). In contrast, DTIC could only induce apoptosis to a similar extent to gold-1a when applied at a relatively high dose (80 mg/kg), whereas when applied at a lower dose (40 mg/kg) only very few apoptotic cells could be seen ().
Figure 2. (A) H & E staining of tumor tissues in different treatment groups. Larger necrotic areas were found in the tumor tissues of gold-1a treated (at both doses) melanoma-bearing mice, compared to those in the solvent control group. DTIC was effective in inducing tumor tissue necrosis only when treated at a relatively high concentration (80 mg/kg), but not at a lower concentration (40 mg/kg). Pictures were taken at 200× magnification. Arrows point to necrotic areas. DTIC-40: DTIC (40 mg/kg); DTIC-80: DTIC (80 mg/kg); Gold-0.125: gold-1a (0.125 mg/kg); Gold-0.25: gold-1a (0.25 mg/kg). (B) In situ cell death detection in the tumor tissues in different treatment groups. The numbers of apoptotic cells in the gold-1a-treated (both 0.125 mg/kg and 0.25 mg/kg) groups were obviously higher compared to those in the solvent control group. DTIC was effective in inducing apoptosis only when treated at a relatively high concentration (80 mg/kg), but not at a lower concentration (40 mg/kg). Pictures shown were taken at 320× magnification. Arrows point to apoptotic nuclei. DTIC-40: DTIC (40 mg/kg); DTIC-80: DTIC (80 mg/kg); Gold-0.125: gold-1a (0.125 mg/kg); Gold-0.25: gold-1a (0.25 mg/kg). Data are shown as mean ± SEM of three mice per treatment group. *p <0.05, compared to solvent control. (C) Immunohistochemical detection of CD31 in the melanoma tissues of mice in different treatment groups. iMVD was significantly lower in the gold-1a (0.25 mg/kg) group, compared to the solvent control group. Pictures shown were taken at 100× magnification. Arrows point to CD31-positive microvessels. DTIC-40: DTIC (40 mg/kg); DTIC-80: DTIC (80 mg/kg); Gold-0.125: gold-1a (0.125 mg/kg); Gold-0.25: gold-1a (0.25 mg/kg). Data are shown as mean ± SEM of three mice per treatment group. *p < 0.05, compared to solvent control.
Gold-1a inhibited angiogenesis in vivo
To investigate whether gold-1a inhibited angiogenesis in vivo, the melanoma tissue sections were immunostained with CD31 antibodies. Fewer numbers of CD31-positive microvessels were found in the melanoma tissues of mice treated with either DTIC or gold-1a, compared to those of mice treated with solvent control. Notably, iMVD in the melanoma tissues of the gold-1a (0.25 mg/kg) group was significantly lower than that of the solvent control group. Although DTIC (when applied at 40 mg/kg or 80 mg/kg) also decreased iMVD, the difference was not significant compared to solvent control. Our data indicated that gold-1a was more effective than DTIC in inhibiting angiogenesis in vivo.
Gold-1a inhibited tube formation by MS1 mouse endothelial cells
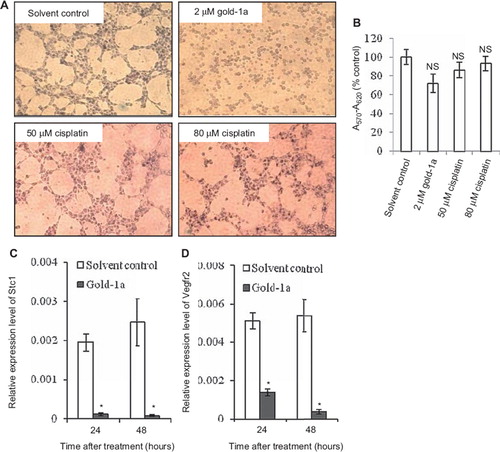
To further confirm that gold-1a posed anti-angiogenic activity, an in vitro angiogenesis assay was employed. Cisplatin was included for comparison due to the structural similarities between cisplatin and gold-1a [Citation8]. Gold(III) is isoelectronic with platinum(II) in cisplatin and forms similar square-planar complexes [Citation8]. Our result showed that gold-1a was more effective than cisplatin in inhibiting tube formation by MS1 cells. As shown in , gold-1a at the dosage of 2 μM significantly inhibited tube formation by MS1 cells, whereas the same phenomenon could not be observed when treated with cisplatin at a dosage up to 80 μM. A noteworthy point is that gold-1a at this dosage did not cause significant toxicity to the cells, as shown by their ability to metabolize MTT into an insoluble purple formazan (). Moreover, cell viability assay showed that treatment of MS1 cells with the compounds at the dosages and for the time period identical to those applied in the tube formation assay did not induce significant cell death ().
Figure 3. (A) In vitro angiogenesis assay. Gold-1a significantly inhibited tube formation by MS1 mouse endothelial cells at 2 μM. In contrast, high concentration of cisplatin (80 μM) did not inhibit tube formation. Data shown are representative pictures from two independent experiments. (B) Treatment with gold-1a or cisplatin at the concentrations the same as those used in the tube formation assay for two hours did not cause significant death of MS1 cells. MTT assay was performed to determine the percentages of viable cells. A570-A620 values obtained from untreated cells were normalized as 100%, and the data were shown as mean ± SEM from three independent experiments. NS, not statistically significant. (C and D) Expression profiles of Stc1 and Vegfr2 in MS1 cells in response to gold-1a treatment, as determined by qRT-PCR. Cells were treated either with solvent control or with 2 (M of gold-1a for 24 or 48 hours. The relative expression levels were compared to those of Gapdh. Data are shown as mean ± SEM from five independent experiments. *p < 0.05, compared to solvent control.
Gold-1a downregulated the expressions of Stc1 and Vegfr2 in MS1 mouse endothelial cells
To evaluate the possible molecular mechanism(s) through which gold-1a inhibited angiogenesis, we studied the expression profiles of genes playing roles in angiogenesis in MS1 mouse endothelial cells in response to gold-1a treatment by qRT-PCR. We found that gold-1a significantly downregulated Stc1 and Vegfr2 in a time-dependent manner (). Stc1 plays roles in angiogenesis [Citation20] and was highly upregulated in an in vitro model of angiogenesis [Citation21]. Vegfr2 is a signal transducer for cancer-related angiogenesis [Citation22].
Discussion
Thus far, gold-1a has been shown to prolong the survival of HCC-bearing rats [Citation10] and NPC metastasis-bearing mice [Citation11], and inhibited tumor growth in mice bearing NPC [Citation12], neuroblastoma [Citation13] and colon cancer [Citation14]. The major mechanism of gold-1a's anti-tumor action was induction of apoptosis [Citation10,Citation12, Citation14,Citation15] and inhibition of tumor cell migration and invasion [Citation11]. In this study, gold-1a has been shown to significantly prolong the survival and inhibit tumor growth in melanoma-bearing mice. More importantly, gold-1a significantly inhibited angiogenesis in vivo. As expected, gold-1a also induced apoptosis in the tumor tissues of the melanoma-bearing mice. The effect was stronger than that of DTIC, the standard chemotherapeutic drug in melanoma. Notably, in the present study, the body weights of gold-1a-treated mice were more or less the same as the solvent control-treated mice. In our previous study, gold-1a also did not cause an evident drop of body weight in HCC-bearing rats and did not increase the plasma aspartate aminotransferase level [Citation10], indicating that there was no observable side-effect of gold-1a. What is more, our previous acute toxicity evaluation revealed that the median lethal dosage of gold-1a was 4.4 mg/kg [Citation23,Citation24], whereas our data showed that melanoma was highly sensitive to gold-1a at 0.25 mg/kg. Moreover, there was no observable damage in the major organs including the kidney, lung, heart, brain and reproductive organs of the mice used in the acute toxicity evaluation study [Citation24]. The difference in the lethal and effective dosages creates a safe therapeutic window for gold-1a and thus warrants gold-1a to be developed as a promising anti-melanoma agent.
Inhibition of angiogenesis is one of the most important cancer therapeutic approaches. We demonstrated that gold-1a exhibited anti-angiogenic activities both in vivo and in vitro. Gold-1a decreased iMVD in the mouse melanoma tissues. In the in vitro angiogenesis assay, the anti-angiogenic action of gold-1a was not due to its cytotoxic activity, since most of the cells remained viable after having exposed to the compound, as demonstrated by the ability of the cells to metabolize MTT, as well as by the corresponding cell proliferation assay. A noteworthy point is that cisplatin at the dosage as high as 80 μM could not cause significant inhibition of tube formation. The detailed molecular mechanism through which gold-1a inhibited angiogenesis is not clear, but we found that Stc1, believed to have a role in angiogenesis [Citation20], was significantly downregulated in a time-dependent manner in response to gold-1a in MS1 cells. Stc1 was highly upregulated in an in vitro model of angiogenesis [Citation21]. Its expression was also closely parallel to that of CD31, an endothelial marker, in a mouse femoral artery ligation model of angiogenesis [Citation21]. Vegfr2, a transmembrane receptor that plays roles in development of endothelial cells [Citation25] was also significantly downregulated by gold-1a in MS1 cells in a time-dependent manner. Vegfr2 was overexpressed in lung, colon, uterus, ovarian and breast cancers [Citation26]. Downregulation of Stc1 and Vegfr2 in endothelial cells might represent part of the molecular events through which gold-1a inhibited angiogenesis.
In summary we have demonstrated that gold-1a could prolong the survival of melanoma-bearing mice. The compound exhibited higher inhibitory effect of melanoma growth in vivo compared to the clinically-used DTIC. It also inhibited angiogenesis both in vivo and in vitro. Gold-1a might potentially be used clinically to treat melanoma patients.
Acknowledgements
This study was supported by grants from the AoE Scheme of UGC (AoE/P-10/01), RGC (HKU7705/ 07M to MCL) of the Hong Kong Special Administrative Region, China. There are no financial disclosures from any authors.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277–300.
- Bellett RE, Mastrangelo MJ, Laucius JF, Bodurtha AJ. Randomized prospective trial of DTIC (NSC-45388) alone versus BCNU (NSC-409962) plus vincristine (NSC-67574) in the treatment of metastatic malignant melanoma. Cancer Treat Rep 1976;60:595–600.
- Cagnoli M, Alama A, Barbieri F, Novelli F, Bruzzo C, Sparatore F. Synthesis and biological activity of gold and tin compounds in ovarian cancer cells. Anticancer Drugs 1998;9:603–10.
- Marcon G, Carotti S, Coronnello M, Messori L, Mini E, Orioli P, . Gold(III) complexes with bipyridyl ligands: Solution chemistry, cytotoxicity, and DNA binding properties. J Med Chem 2002;45:1672–7.
- Ward JR. Role of disease-modifying antirheumatic drugs versus cytotoxic agents in the therapy of rheumatoid arthritis. Am J Med 1988;85:39–44.
- Simon TM, Kunishima DH, Vibert GJ, Lorber A. Cellular antiproliferative action exerted by auranofin. J Rheumatol Suppl 1979;5:91–7.
- Simon TM, Kunishima DH, Vibert GJ, Lorber A. Inhibitory effects of a new oral gold compound on HeLa cells. Cancer 1979;44:1965.
- Tiekink ER. Gold derivatives for the treatment of cancer. Crit Rev Oncol Hematol 2002;42:225–48.
- Sun RW, Che CM. The anti-cancer properties of gold(III) compounds with dianionic porphyrin and tetradentate ligands. Coord Chem Rev 2009;253:1682–91.
- Lum CT, Yang ZF, Li HY, Sun RW, Fan ST, Poon RT, . Gold(III) compound is a novel chemo-cytotoxic agent for hepatocellular carcinoma (HCC). Int J Cancer 2006;118:1527–38.
- Lum CT, Liu X, Sun RW, Li XP, Peng Y, He ML, . Gold(III) porphyrin 1a inhibited nasopharyngeal carcinoma metastasis in vivo and inhibited cell migration and invasion in vitro. Cancer Lett 2010;294:159–66.
- To YF, Sun RW, Chen Y, Chan VS, Yu WY, Tam PK, . Gold(III) porphyrin complex is more potent than cisplatin in inhibiting growth of nasopharyngeal carcinoma in vitro and in vivo. Int J Cancer 2009;124:1971–9.
- Li W, Xie Y, Sun RW, Liu Q, Young J, Yu WY, . Inhibition of Akt sensitizes neuroblastoma cells to gold(III) porphyrin 1a, a novel antitumour drug induced apoptosis and growth inhibition. Br J Cancer 2009;101:342–9.
- Tu S, Sun RW, Lin MC, Cui JT, Zou B, Gu Q, . Gold(III) porphyrin complexes induce apoptosis and cell cycle arrest and inhibit tumor growth in colon cancer. Cancer 2009; 115:4459–69.
- Wang Y, He QY, Sun RW, Che CM, Chiu JF. GoldIII porphyrin 1a induced apoptosis by mitochondrial death pathways related to reactive oxygen species. Cancer Res 2005;65:11553–64.
- Nyberg P, Salo T, Kalluri R. Tumor microenvironment and angiogenesis. Front Biosci 2008;13:6537–53.
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature 2000;407(6801):249–57.
- Che CM, Sun RW, Yu WY, Ko CB, Zhu N, Sun H. Gold(III) porphyrins as a new class of anticancer drugs: Cytotoxicity, DNA binding and induction of apoptosis in human cervix epitheloid cancer cells. Chem Commun (Camb) 2003;14:1718–9.
- Lens MB, Eisen TG. Systemic chemotherapy in the treatment of malignant melanoma. Expert Opin Pharmacother 2003;4:2205–11.
- Kahn J, Mehraban F, Ingle G, Xin X, Bryant JE, Vehar G, . Gene expression profiling in an in vitro model of angiogenesis. Am J Pathol 2000;156:1887–900.
- Zlot C, Ingle G, Hongo J, Yang S, Sheng Z, Schwall R, . Stanniocalcin 1 is an autocrine modulator of endothelial angiogenic responses to hepatocyte growth factor. J Biol Chem 2003;278:47654–9.
- Shibuya M. Differential roles of vascular endothelial growth factor receptor-1 and receptor-2 in angiogenesis. J Biochem Mol Biol 2006;39:469–78.
- Sun RW, Ma DL, Wong EL, Che CM. Some uses of transition metal complexes as anti-cancer and anti-HIV agents. Dalton Trans 2007;43:4884–92.
- Sun RW, Che CM. The anti-cancer properties of gold(III) compounds with dianionic porphyrin and tetradentate ligands. Coord Chem Rev 2009;253:1682–91.
- Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, . Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature 1995;376:62–6.
- Giatromanolaki A, Koukourakis MI, Sivridis E, Chlouverakis G, Vourvouhaki E, Turley H, . Activated VEGFR2/KDR pathway in tumour cells and tumour associated vessels of colorectal cancer. Eur J Clin Invest 2007;37:878–86.
