Abstract
Background. The diagnosis and treatment of cancer may cause clinically significant and persistent psychological morbidity. The objective of this study was to determine the short-term effect of a six week exercise intervention on anxiety and depression in cancer patients undergoing chemotherapy (The ‘Body & Cancer’ trial). Methods. Two hundred and nine self-referred patients (52 males, 157 females, mean age 47 years) were randomised into an intervention group and a waiting-list control group. Anxiety and depression was measured by the Hospital Anxiety and Depression Scale. Results. At baseline, 23.5% and 11.5% of the population scored >8 on the HADS and were classified as suspicious or definite cases of anxiety and depression, respectively. Adjusted for baseline score, disease and demographic covariates the estimated intervention effect showed improvement at six weeks for depression of −0.7 points (95% confidence interval [CI] −1.27 to −0.14, p = 0.0153). No significant effect was seen on anxiety. Further subanalysis, including only suspicious or definite cases of depression, resulted in an estimated intervention effect of −2.53 points (95% CI, −0.64 to −0.42, p = 0.021). Conclusion. Anti-depressant effects could be caused by exercise in self-referred cancer patients undergoing chemotherapy. Dedicated trials and follow-up studies are needed to clarify the optimal duration and content of exercise interventions to meet the needs of clinically depressive or anxious patients.
New evidence confirms that a cancer diagnosis prompts a notable increased risk for the development of clinically significant psychological morbidity that can persist for months or even years following successful treatment [Citation1,Citation2]. Although prevalence estimates vary widely evidence suggests that anxiety and depression are among the most common symptoms experienced by cancer patients [Citation3–6]. Possible causes of anxiety and depression for patients undergoing treatment may include concerns about disease progression and disruptions in social life as well as the presence of unpleasant symptoms and/or adverse effects of treatment. Another possible related consequence of cancer treatment is physical inactivity, which in itself is associated with diminished psychological well-being and may lead to higher symptom burden and ultimately poorer survival [Citation7–11]. Systematic reviews and meta-analyses of controlled trials on physical activity in cancer patients during treatment [Citation12,Citation13] conclude that physical activity improves cardiorespiratory fitness, symptoms and physiological effects. Thus, in an attempt to minimise morbidity and enhance quality of life (QOL), cancer patients are advised to sustain or increase their physical activity level [Citation14]. However, only a few experimental studies have attempted to identify the effect of physical activity on psychological well-being, and results are inconsistent [Citation15–20] leaving only weak evidence of a consistent positive effect of physical activity during treatment on anxiety and depression [Citation12].
In 2001, we initiated the ‘Body & Cancer’ trial assessing the effect of a multimodal group exercise intervention, on fatigue, physical capacity, general well-being, physical activity, and QOL in patients undergoing adjuvant chemotherapy or treatment for advanced cancer [Citation21]. The ‘Body & Cancer’ project was designed to provide body-focussed and socially orientated efforts to support Oncological and Haematological cancer patients at different stages of the disease (evidence vs. no evidence of residual disease), and in varying cytostatic treatment regimes. The project was developed and implemented as a clinical controlled trial by a multidisciplinary research group (physicians, nurses, physiotherapists, psychologist and sociologist) at the Copenhagen University Hospital. Data regarding the effects of the intervention on fatigue, health-related quality-of-life and physical capacity have been published previously [Citation21]. Previous publications furthermore include qualitative studies regarding patients’ experiences of transformation in fatigue [Citation22], development of group cohesion [Citation23] and changes in psychological well-being in advanced disease cancer patients [Citation24]. In the present post-hoc analysis we aimed at analysing the psychological benefits of the intervention, with special emphasis on its effect on anxiety and depression. Thus we tested the hypothesis that a supervised, combined high- and low-intensity exercise intervention, as an adjunct to conventional care, could reduce psychological morbidity (anxiety and depression) in a sample of male and female cancer patients undergoing adjuvant chemotherapy or treatment for advanced disease.
Methods
‘Body & Cancer’ trial design and patients
Briefly, ‘Body & Cancer’ was a waiting-list randomised controlled trial. The objective was to investigate the effect of a six-week intervention comprising high intensity cardiovascular and resistance training, relaxation and body awareness training, and massage, nine hours weekly for six weeks in addition to conventional care (). During heavy resistance training, patients trained at 85–95% of one repetition maximum (1RM) corresponding to six metabolic equivalents (METs; American College of Sports Medicine). The fitness training involved 10-min interval efforts on stationary bicycles, with an intensity of 150–250 W corresponding to 10.5 METs or 85–95% of each patient's maximum heart rate. Warm-up and cool-down exercises comprised dynamic actions with the large muscle groups (eight METs), stretching, and coordination training (2.5 METs). In total the high intensity activities corresponded to 33 MET-hours per week. The patients carried out the training in mixed groups (male and female) of seven to nine patients in each. Specially trained physiotherapists and nurses supervised the intervention, and participated in the physical training with the patients. The programme took place in a specially designed workout room located at the hospital. A carefully selected choice of equipment intended to provide a professional yet relaxed environment to distract from the sterile hospital environment. Due to a lack of locker rooms, patients had to change clothes in a common area located outside of the training room. This area included a ‘lounge’ where patients were served coffee, tea, fruit and raisins.
Table I. Multimodal exercise intervention, weekly schedule. Values are hours.
Two hundred and nine cancer patients undergoing chemotherapy participated in a waiting-list randomised controlled exercise trial. Patients were randomised by computer (Clinical Internet Trial Management System, CITMAS) and assigned to either a structured and supervised group-based exercise programme (nine hours weekly for six weeks) or a waiting-list control group. All patients in addition received standard care from physicians and nurses. Eligible patients met the following criteria: a diagnosis of cancer; completed at least one cycle of chemotherapy for advanced disease or as adjuvant treatment; a WHO performance status of 0 (i.e. asymptomatic) or 1 (i.e. symptomatic but completely ambulatory); and aged 18–65 years. Exclusion criteria were brain- or bone metastases, thrombocytopenia (<50 > 109/l), myocardial infarction within the last three months or uncontrolled hypertension (diastolic pressure >95 mmHg). The control group was allowed to freely increase physical activity and were in no way restrained from participation in existing supportive care offers. The design and main findings of the ‘Body & Cancer’ trial are described in detail elsewhere [Citation21,Citation25].
Outcome assessment
Data on depression and anxiety were prospectively collected in the ‘Body & Cancer’ trial. All participants completed the Hospital Anxiety and Depression Scale (HADS) before randomisation. The assessment was repeated post intervention (week 6). The HADS comprises two scales: The HADS-Anxiety Scale (HADS-A) and the HADS-Depression Scale (HADS-D). While the HADS-A measures generalised, autonomic anxiety, and indicates the state of physiological and emotional hyper-arousal marked by high muscle tension and strong feelings of subconscious and uncontrollable fear or anger [Citation26], the HADS-D measures anhedonia, understood as a complete loss of interest or pleasure, described as ‘exclusion from the pleasure dome’ [Citation27]. The HADS consists of 14 items, seven on the HADS-A and seven on the HADS-D. Each item is scored on a four-point scale from 0 (not present) to 3 (considerable), and the item scores are added, giving HADS-D and HADS-A subscale scores from 0 (minimum symptom load) to 21 (maximum symptom load). One of the main purposes of the HADS is to identify clinical cases of anxiety disorders and depression among medical patients in non-psychiatric hospital clinics [Citation28]. Scores of 11 or more on either sub-scale was considered to indicate those who were likely to be a ”definite case” while scores of 8–10 represented “suspicious cases” and 0–7 “non-cases” of anxiety or depression, respectively. These cut-off scores have been shown to give an optimal balance between sensitivity and specificity on receiver operating characteristic curves [Citation28]. Demographic and behavioural data were collected by self-report, and medical data were drawn from records. Leisure time physical activity level was explored by self-report questionnaire. The participants were classified as: (I) sedentary (completely inactive); (II) walking or cycling for pleasure; (III) regular, physical exercise at least three hours/week; or (IV) intense physical activity more than four hours/week (athletic).
Statistical analysis
Power calculations were performed on fatigue, which was the primary outcome measure in the ‘Body & Cancer’ trial [Citation21]. Baseline comparisons were performed using univariate analyses of variance for continuous variables. For categorical measures likelihood ratio based χ2 test statistics for symmetry and marginal homogeneity were used to evaluate the potential changes. The main analysis was undertaken post hoc and examined whether significant differences existed between the control and intervention group in anxiety and depression measures (HADS). This was accomplished by performing a forward stepwise regression analysis using differences in outcomes between baseline and six week as the dependent variable in a general linear model (GLM). The stepwise procedure began by identifying the covariate that is most strongly related to the dependant variable. The next strongest related covariate is then selected after controlling for the first covariate, etc. The variable: intervention/control group was fixed and adjusted for the following covariates: gender, age, cohabitation, educational level, baseline outcome score, current smoker, relative change in haemoglobin, aerobic capacity (VO2max), muscle strength (1RM knee extension), and the four disease-related covariates; diagnosis, no evidence of disease (NED), evidence of disease (ED) and relapse of disease. Potential effect modifications (interactions) of the five disease related covariates and their influence on the estimated mean difference were also tested.
Furthermore, we performed subgroup analysis to assess only participants whose baseline anxiety and depression scores were higher than seven (proposed as the optimal cut-off point for the identification of cases on both subscales). For all analyses, we tested with a level of significance at p ≤ 0.05 and used the intention-to-treat principle. Available data for participants with missing data were included under the missing at random assumption. Effect size (ES) was calculated by the mean difference divided by the pooled standard deviation, the Root Mean Square Error (RMSE) estimated from the general linear model (GLM). Clinically important changes were estimated using Cohen's guidelines, whereby a value of 0.2 denotes a small, 0.5 a medium, and 0.8 a large effect size [Citation29]. Participants classified as lost to follow-up (n = 60) were compared to the study group (n = 209) for baseline demographic data using ordinary t-test and χ2 test for categorical variables. Data were entered into Excel using Microsoft Office 2000 Professional for Windows XP and statistical analyses were carried out using SAS for Windows (version 9.3.2).
Results
Participants
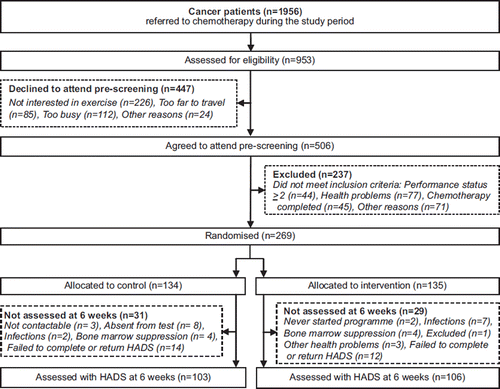
Of a total of 1956 cancer patients between 18–65 years of age referred to chemotherapy at the Oncological and Haematological departments at the Copenhagen University Hospital (Rigshospitalet) or the Herlev Hospital, 953 cancer patients were assessed for eligibility. Of these, 269 patients agreed to participate and met the inclusion criteria (). On the primary outcomes variables (HADS-A and HADS-D) we obtained post-intervention data after six weeks from 106 participants in the intervention group (78.5%) and 103 in the control group (76.7%).
The intervention group's adherence rate for the supervised exercise sessions was 73.1% (mean 18 days of 24 training days, range 5–24). The groups were balanced at baseline and and show the demographic and medical characteristics of the participants. Participants had a mean age of 47.5 years (range 20–65, median = 48). The intervention group included 22 men and 84 women, and 30 men and 73 women participated in the control group. There were no statistical differences in the disease variables: diagnoses, relapsed disease, haemoglobin, days since diagnosis, NED, and ED were observed between the intervention- and the control group at baseline (). Participants from the intervention group and the control group had received a mean of 2.5 and 2.9 cycles of chemotherapy respectively prior to the study period and a mean of 1.9 and 1.8 cycles respectively during the six-week study period. The number of chemotherapy cycles did not differ significantly between the two groups ().
Table II. Demographic characteristics for all patients (n=209) and by group assignment. Values are numbers (percentages) of patients unless stated otherwise.
Table III. Medical characteristics for all patients (n = 209) and by group assignment. Values are numbers (percentages) of patients unless stated otherwise.
Baseline characteristics: Prevalence of patient-rated psychological morbidity
The mean score on the HADS-A, for the whole population, at baseline was 5.35 (SD 3.59 range 0–19), and the mean score on the HADS-D for the whole population at baseline was 3.76 (SD 3.12 range 0–14). There was a significant correlation between the HADS-A and the HADS-D scores at baseline (p = 0.575; p < 0.001). Using a threshold score of 8, 23.4% (n = 49) of the participants were identified as at least ‘doubtful’ (moderate) cases for anxiety, and 11.5% (n = 24) as suspicious or definite cases of depression. However, the prevalence of HADS-defined depression was higher among those who reported being married or being in a relationship than among those who were single (p = 0.005) and higher among those who reported being physically inactive in comparison with those who were physically active (p < 0.001). There was no association between prevalence of HADS-defined anxiety disorder and any of the remaining background variables. In addition, there was no significant difference in the distribution of anxiety and depression cases between the intervention and control group at baseline.
Follow-up characteristics: Changes in patient-rated psychological morbidity
presents patient-rated anxiety and depression measured at baseline and after six weeks for the intervention and the control groups separately and for all participants. We found a significant effect for depression from baseline to six weeks in favour of the intervention group. The intervention group had a reduced depression score of an estimated mean difference of −0.7 points compared to the control group (p = 0.0153). Changes in anxiety numerically favoured the intervention group, but did not reach statistical significance (). The changes in anxiety and depression were significantly correlated (p = 0.465; p < 0.001). The attrition analysis showed that, amongst participants classified as lost to follow-up (n =60), there were significant more non-cases (score <8) of anxiety (p = 0.0002) and depression (p = 0.0228) in comparison the study group (n = 209). As a matter of fact, not a single individual in the attrition group scored above 8 on either subscale. The attrition group was comparable to the follow-up group in terms of gender (p = 0.1271) and age (p = 0.4782).
Table IV. Hospital Anxiety and Depression Scale (HADS) outcome variables and intervention effect estimates (95% confidence intervals) for all patients.
Subgroup analysis of suspicious or definite cases of psychological morbidity
presents patient-rated anxiety and depression measured at baseline and after six weeks, for participants with HADS-defined anxiety (n = 49) and depression (n = 24), i.e. participants scoring 8 or above in either of the subscales at baseline. Using this classification, we found a significant effect for depression from baseline to six weeks in favour of the intervention group. Participants in the intervention group defined by the HADS as borderline or definite cases of depression reduced their depression score with a mean difference of −2.53 points compared to their counterparts in the control group (p = 0.021). In comparison, participants in the intervention group being defined by the HADS-D as non-cases did not obtain a significant reduction in their depression score compared to the control group (p = 0.11).
Table V. Hospital Anxiety and Depression Scale (HADS) outcome variables and intervention effect estimates (95% confidence intervals) for patients with HADS-defined anxiety and depression at baseline (scores ≥8).
Discussion
This is the first study to explicitly and specifically investigate the psychological effect of supervised, group-based multimodal exercise in cancer patients undergoing chemotherapy irrespective of gender, diagnosis and prognosis. Consistent with our hypothesis, the participants in the exercise intervention group significantly reduced their level of depression. However, contrary to our hypothesis, no significant intervention effect was seen on the level of anxiety, regardless of the severity of anxiety symptoms. Thus, this finding indicates, that while multimodal exercise may serve as an antidepressant, it seems to do little to reduce anxiety. This is especially noteworthy both clinically and methodologically given the debate of whether multi-symptom approaches such as the HADS mostly assesses negative affectivity, a nonspecific form of distress, [Citation30,Citation31] or whether they should still be used as two-dimensional [Citation32]. In fact, this study confirms that anxiety and depression, although highly correlated, methodologically and clinically are two distinct symptoms, and that this may become particularly apparent in an attempt to estimate and explain the psychological effects of exercise on cancer patients.
The differentiation and discrimination between an anxiolytic and the antidepressant effect of exercise during cancer treatment is in accordance with own previous qualitative studies [Citation25,Citation33]. Phenomenological and narrative analysis of diaries of patients with advanced disease [Citation25] who exercised while undergoing treatment, showed that while the intervention provided situations for each participant to experience pleasure and excitement, allowing the individual to gain new hope and, the intervention was less able to overshadow the uncertainty of the illness. This thinking is furthermore in line with our most recent publication [Citation21] showing that participants significantly reduced fatigue, no significant effect could be found in overall (global) QOL. Together these studies support the assumption that cancer patients tend to experience symptoms in clusters rather than in isolation [Citation34]. Depression is known to frequently co-occur with fatigue in cancer patients [Citation35,Citation36], whereas QOL may be more determined by generalised anxiety (including feelings of worry, apprehension, and dread) [Citation31], which may be affected to a lesser degree by exercise.
Although the intervention in the present study did not significantly improve anxiety, the overall results still appear more favourable than results achieved in comparable studies. In a recent study [Citation37] on the effects of aerobic and resistance exercise in breast cancer patients (n = 242) receiving adjuvant chemotherapy, neither anxiety nor depression improved. Similarly, Cadmus et al. [Citation38] evaluated the effect of a six-month home-based exercise programme in newly diagnosed breast cancer patients (n = 50) and found no significant effect of the intervention on psychological morbidity. Finally, Mock et al. [Citation15] showed an effect of a six-week walking exercise programme on anxiety but not depression in women undergoing adjuvant radiation therapy treatment for breast cancer (n = 46). In light of these studies, possible explanations for the positive effects achieved in our study may include a more intense, structured and professionally supervised exercise regime.
Several issues must be considered when determining the meaningfulness of the obtained change in depression. No guideline is available for the HADS in order to interpret if these findings translate into a clinically important difference (CID). However, according to the scoring manuals for HADS, a move across suggested cut-off scores should be considered a clinically significant change. In order not to overestimate the clinical significance of score differences at pre- and post-testing, a change of 2 points can be considered to be a small to moderate clinically significant change, whereas a difference of 5 points or higher is considered to be a moderate to large clinically significant change. Thus, the magnitude of the effect of the intervention on patients with HADS-defined depression (mean −5.75, SD 2.31) suggests that the intervention has clinical significance. Using the original criteria proposed by Cohen [Citation29] for calculating effect sizes, the magnitude of the effect of the intervention on depression (0.36) may be described for the whole group as small to moderate, whereas the effect on patients defined at baseline as subclinical or clinical cases of depression (0.99) may be interpreted as large, indicating substantial clinical relevance of the present intervention's effect. In comparison, the weighted mean effect size (95% confidence interval) for controlled physical activity trials during treatment intervention studies is 0.09 (−0.23, 0.42) for depression and 0.22 (−0.11, 0.54) for anxiety [Citation12]. However, conventional statistical approaches, such as the guidelines proposed by Cohen, tell us only little about the clinical psychotherapeutic efficacy of the intervention and an optimal method for deriving clinical significance remains to be determined [Citation39,Citation40].
Early recognition and effective treatment of depression in cancer patients are of clinical importance, especially in light of new evidence [Citation2] showing increased hospitalisation rates for depression years following a cancer diagnosis and completion of primary treatment. The HADS baseline scores demonstrate that the population in general reported fairly low levels of distress, and thus did not suffer from clinical anxiety and/or depression. Therefore the obtained effect is likely to be caused by considerable changes in patients who scored relatively high at baseline. Thus, participants characterised as cases of depression in the control group reduced their depression score by −2.5 in average compared to an average reduction of −6.6 among cases of depression in the intervention group.
However, the original HADS cut-off scores were conceived for evaluating primary care patients, and it has recently been argued [Citation41,Citation42] that lower thresholds may be required in the clinical care of cancer patients. In a recent study [Citation42] based on data from 689 cancer patients assessed during their first days of in-patient treatment, the authors found that for clinical purposes the optimal thresholds should be ≥3 for anxiety (HADS-A) and ≥2 for depression (HADS-D) and ≥6 for overall psychological morbidity (HADS-T). Applying these thresholds to the present study, results in baseline prevalence rates of 76.1% for anxiety and 74.2% for depression. Furthermore, lowering the thresholds result in significant improvements from the intervention on depression (95% CI −1.44 to −0.009, p = 0.047, ES = 0.32), while the effect of the intervention on overall psychological morbidity and anxiety remains insignificant. However, in comparison with the patients in the Singer et al. study [Citation42], most of the patients in our study were outpatients not recently admitted for hospital treatment (median days since diagnosis was 80). For this reason, and since we used the HADS for research and not for clinical screening purposes, it seemed reasonable in the present study to preserve the original thresholds of ≥8 as proposed by Zigmond and Snaith [Citation43] for primary care patients.
In perspective, the diagnosis and treatment of cancer present a challenge for the patients’ psychological adjustment and ability to cope and therefore must include not only medical procedures but also mental health care [Citation42]. In perspective, exercise interventions need to be evaluated in combination with other psychosocial approaches used to manage anxiety and depression. Based largely on consensus, the NCCN Clinical Practice Guidelines for Distress Management recommends the use of psychotherapy in combination with antidepressant and/or anxiolytic medication for patients with mood or anxiety disorders [Citation44]. Randomised clinical trials are needed that explicitly test whether the combination of pharmacotherapy, exercise and psychotherapy is better than separate approaches in managing anxiety and depression. Furthermore, it has been shown that psychological distress after cancer in itself may place patients at risk of reduced physical activity [Citation45]. Thus, it is imperative to continue to promote exercise early in the cancer trajectory and invite patients to enter a positive and self-increasing process whereby they can sustain their physical activity while undergoing treatment. However, cancer patients should be encouraged to engage in physical activity also because of the protective effect of exercise on psychological well-being and not primarily and solely because it may improve their chances of disease-free survival, as the latter may pose a responsibility on the individual that can lead to self-blame in cases of disease recurrence. By emphasising the anti-depressant potentials of physical activity, clinicians may help patients to experience exercise as a privilege and a goal in itself, which most likely will promote sustainment.
Methodological considerations
This is the first randomised, controlled study that evaluated the psychological effect of a supervised, combined high- and low-intensity exercise intervention, and incorporated heavy resistance and relaxation training in conjunction with cardiovascular training for cancer patients undergoing cytostatic treatment. It never was the intention of the study, nor possible, to differentiate or isolate the potential effect of each of the intervention component alone. The intervention was designed as a total package involving group belonging/peer support, high- and low physical activities and encouragement from coaches/instructors. The strengths of our study include the randomised design that allowed for a concurrent comparison group and the use of a reliable and valid tool (the HADS) for identifying and quantifying the effects of multimodal exercise on the two most common forms of psychological disturbances in adult cancer patients. Another advantage includes the ability to adjust for multiple demographic, physiological and medical factors that may affect psychological well-being such as gender, age, physical fitness and disease status. Finally, in contrast to most other studies, we included both male and female patients and patients with advanced disease. We realise that the complexity of the study population regarding age and cancer diagnosis can make interpretation of study results difficult to assess. However, we aimed at investigating if a broad spectrum of cancer patients, as seen in the clinic, could comply to and benefit from a well-defined training programme. A limitation of the study is the lack of long-term follow-up, which was prevented due to the wait-list control design that allowed participants to undertake the intervention immediately after the six week intervention period. Another limitation is the unequal numbers of female and male patients and the small number of patients in some diagnosis groups, which did not allow for cross-gender and cross-cancer comparisons to be made concerning the psychological response to the intervention. Furthermore, the study lacked access to valid data on patients’ potential use of psychopharmacological drugs, which could help to further explain the prevalence of distress in relation to the effect of the intervention. Finally, self-reference of participants resulted in a sample of patients who were overtly motivated to engage in group based physical activity. Moreover the participants were relatively young and well-educated not reflecting the entire background population of cancer patients undergoing chemotherapy.
Conclusion
In conclusion, the present study, based on analyses of data from the ‘Body and Cancer’ trial, suggest that a multimodal exercise intervention could reduce depression but not anxiety in self-referred, highly motivated cancer patients undergoing treatment. Strategies for future research include limiting enrolment to survivors who report clinically significant levels of psychological morbidity and targeting patient subgroups known to be at particular risk for psychological impairment/distress. This study provides rationale for promoting non-pharmacological, complementary, and combined exercise intervention, including the potential for overcoming clinically significant depression in cancer patients undertaking cytostatic treatments.
Acknowledgements
This research was supported, in part, by grants from The Lundbeck Foundation (FP03, FP1, FP071/01, FP126/99), The Novo Nordisk Foundation, The Egmont Foundation (831-2083), and The Danish Cancer Society (PP03027, PP04001, PP01001), The Svend Andersen Foundation and The Aase & Ejnar Danielsen Foundation (104722, 103363). Trial registration Current Controlled trials ISRCTN05322922.
The authors work was independent of the funders. We thank the participants in the Body & Cancer Study.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Foster C, Wright D, Hill H, Hopkinson J, Roffe L. Psychosocial implications of living 5 years or more following a cancer diagnosis: A systematic review of the research evidence. Eur J Cancer Care (Engl) 2009;18:223–47.
- Dalton SO, Laursen TM, Ross L, Mortensen PB, Johansen C. Risk for hospitalization with depression after a cancer diagnosis: A nationwide, population-based study of cancer patients in Denmark form 1973 to 2003. J Clin Oncol 2009; 27:1440–5.
- Onelöv E, Steineck G, Nyberg U, Hauksdóttir A, Kreicbergs U, Henningsohn L, . Measuring anxiety and depression in the oncology setting using visual-digital scales. Acta Oncol 2007;46:810–6.
- Breitbart WS, Alici Y. Psycho-oncology. Harv Rev Psychiatry 2009;17:361–76.
- Pirl WF. Evidence report on the occurrence, assessment, and treatment of depression in cancer patients. J Natl Cancer Inst Monogr 2004;32:32–9.
- Massie MJ. Prevalence of depression in patients with cancer. J Natl Cancer Inst Monogr 2004;32:57–71.
- Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. JAMA 2005;293:2479–86.
- Meyerhardt JA, Giovannucci EL, Holmes MD, Chan AT, Chan JA, Colditz GA, . Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol 2006;24:3527–34.
- Meyerhardt JA, Heseltine D, Niedzwiecki D, Hollis D, Saltz LB, Mayer RJ, . Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Clin Oncol 2006;24:3535–41.
- Meyerhardt JA, Giovannucci EL, Ogino S, Kirkner GJ, Chan AT, Willett W, . Physical activity and male colorectal cancer survival. Arch Intern Med 2009;169:2102–8.
- Ogunleye AA, Holmes MD. Physical activity and breast cancer survival. Breast Cancer Res 2009;11:106 (doi:10.1186/bcr2351).
- Schmitz KH, Holtzman J, Courneya KS, Mâsse LC, Duval S, Kane R. Controlled physical activity trials in cancer survivors: A systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 2005;14:1588–95.
- Markes M, Brockow T, Resch KL. Exercise for women receiving adjuvant therapy for breast cancer. Cochrane Database Syst Rev 2006;18:CD005001.
- Sharp DM, Walker MB, Chaturvedi A, Upadhyay S, Hamid A, Walker AA, . A randomised, controlled trial of the psychological effects of reflexology in early breast cancer. Eur J Cancer 2010;46:312–22.
- Mock V, Dow KH, Meares CJ, Grimm PM, Dienemann JA, Haisfield-Wolfe ME, . Effects of exercise on fatigue, physical functioning, and emotional distress during radiation therapy for breast cancer. Oncol Nurs Forum 1997; 24:991–1000.
- Segar ML, Katch VL, Roth RS, Garcia AW, Portner TI, Glickman SG, . The effect of aerobic exercise on self-esteem and depressive and anxiety symptoms among breast cancer survivors. Oncol Nurs Forum 1998;25:107–13.
- Dimeo FC, Stieglitz RD, Novelli-Fischer U, Fetscher S, Keul J. Effects of physical activity on the fatigue and psychologic status of cancer patients during chemotherapy. Cancer 1999;85:2273–7.
- Mock V, Pickett M, Ropka ME, Muscari Lin E, Stewart KJ, Rhodes VA, . Fatigue and quality of life outcomes of exercise during cancer treatment. Cancer Pract 2001;9:119–27.
- Burnham TR, Wilcox A. Effects of exercise on physiological and psychological variables in cancer survivors. Med Sci Sports Exerc 2002;34:1863–7.
- Courneya KS, Friedenreich CM, Sela RA, Quinney HA, Rhodes RE, Handman M. The group psychotherapy and home-based physical exercise (group-hope) trial in cancer survivors: Physical fitness and quality of life outcomes. Psychooncology 2003;12:357–74.
- Adamsen L, Quist M, Andersen C, Møller T, Herrstedt J, Kronborg D, . Effect of a multimodal high intensity exercise intervention in cancer patients undergoing chemotherapy: randomised controlled trial. BMJ 2009;339:b3410.
- Adamsen K, Midtgaard J, Roerth M, Andersen C, Quist M, Moeller T. Transforming the nature of fatigue through exercise: Qualitative findings from a multidimensional exercise programme in cancer patients undergoing chemotherapy. Eur J Cancer Care 2004;13:362–70.
- Midtgaard J, Roerth M, Stelter R, Adamsen L. The group matters: An explorative study of group cohesion and quality of life in cancer patients participating in physical exercise intervention during treatment. Eur J Cancer Care 2006; 15:25–33.
- Midtgaard J, Stelter R, Rørth M, Adamsen L. Regaining a sense of agency and shared self-reliance: The experience of advanced disease cancer patients participating in a multidimensional exercise intervention while undergoing chemotherapy – analysis of patient diaries. Scand J Psychol 2007;48: 181–90.
- Midtgaard J, Stelter R, Rørth M, Adamsen L. Regaining a sense of agency and shared self-reliance: The experience of advanced disease cancer patients participating in a multidimensional exercise intervention while undergoing chemotherapy-analysis of patient diaries. Scand J Psychol 2007;48: 181–90.
- Dunbar M, Ford G, Hunt K, Der G. A confirmatory factor analysis of the Hospital Anxiety and Depression Scale: Comparing empirically and theoretically derived structures. Br J Clin Psychol 2000;39:79–94.
- Snaith P. Anhedonia: Exclusion from the pleasure dome. BMJ 1992;305:134.
- Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale: An updated literature review. J Psychosom Res 2002;52:69–77.
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd. Hillsdale, NJ: Erlbaum; 1988.
- Clark LA, Watson D. Theoretical and empirical issues in differentiating depression from anxiety. Becker J, Kleinman A. Psychosocial aspects of depression. Hillsdale, NJ: Erlbaum: 1991. 39–65.
- Watson D, Weber K, Assenheimer JS, Clark LA, Strauss ME, McCormick RA. Testing a tripartite model: I. Evaluating the convergent and discriminant validity of anxiety and depression symptom scales. J Abnorm Psychol 1995;104:3–14.
- Hermann C. International experiences with the Hospital Anxiety and Depression Scale: A review of validation data and clinical results. J Psychosom Res 1997;42:17–41.
- Midtgaard J, Rørth M, Stelter R, Adamsen L. The group matters: An explorative study of group cohesion and quality of life in cancer patients participating in physical exercise intervention during treatment. Eur J Cancer Care 2006; 15:25–33.
- Miaskowsky C, Dodd M, Lee K. Symptom clusters: The new frontier in symptom management research. J Natl Cancer Inst Monogr 2004;32:17–21.
- Gaston-Johansson F, Fall-Dickinson JM, Bakos AB, Kennedy MJ. Fatigue, pain, and depression in preautotransplant breast cancer patients. Cancer Pract 1999;7:204–7.
- Donovan KA, Jacobsen PB. Fatigue, depression, and insomnia: Evidence for a symptom cluster in cancer. Semin Oncol Nurs 2007;23:127–35.
- Courneya KS, Sellar CM, Stevinson C, McNeely ML, Peddle CJ, Friedenreich CM, . Randomized controlled trial of the effects of aerobic exercise on physical functioning and quality of life in lymphoma patients. J Clin Oncol 2009;27:4605–12.
- Cadmus LA, Salovey P, Yu H, Chung G, Kasl S, Irwin ML. Exercise and quality of life during and after treatment for breast cancer: results of two randomized controlled trials. Psychooncology 2009;18:343–52.
- Jacobson NS, Truax P. Clinical significance: A statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol 1991;59:12–9.
- Sloan JA, Cella D, Frost MH, Guyatt G, Osoba D. Quality of life III: Translating the science of quality-of-life assessment into clinical practice – an example-driven approach for practicing clinicians and clinical researchers. Clin Ther 2003;25.
- Morse R, Kendell K, Barton S. Screening for depression in people with cancer: The accuracy of the hospital anxiety and depression scale. Clin Eff Nurs 2005;9:88–196.
- Singer S, Kuhnt S, Götze H, Hauss J, Hinz A, Liebmann A, . Hospital anxiety and depression scale cutoff scores for cancer patients in acute care. Br J Cancer 2009;100:908–12.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361–70.
- National Comprehensive Cancer Network. NCCN practice guidelines for the management of psychosocial distress. Oncology (Williston Park) 1999;13:113–47.
- Chambers SK, Lynch BM, Aitken J, Baade P. Relationship over time between psychological distress and physical activity in colorectal cancer survivors. J Clin Oncol 2009;27:1600–6.