Abstract
Background. Immunomagnetic EpCAM based methods are used to enrich circulating tumor cells (CTCs) in metastatic breast cancer (mBC) patients. EpCAM negative CTCs may be missed. We addressed the question of the reliability of an EpCAM dependent assay to enrich CTCs. Methods. To elucidate this issue, our study has been designed to assess two different CTC enrichment technologies (i) in EpCAM positive (+) and EpCAM negative cell lines and (ii) in mBC patients in dependency on their respective EpCAM expression. These two technologies encompass one anti-EpCAM immunomagnetic enrichment technology, MACS HEA MicroBeads® (MACS), and one EpCAM independent density centrifugation method, OncoQuick® plus (OQ+). Furthermore, the coherence between EpCAM expression in the primary tumor tissue of mBC patients and the CTC detection rates in the corresponding patients is analyzed. Results. (i) MACS recovered significantly more EpCAM (+) than EpCAM (−) tumor cells (p < 0.001) in spiked blood samples. With OQ+ no significantly different recovery rates between EpCAM (+) and EpCAM (−) tumor cells (p = 0.796) were detected. (ii) In mBC patients MACS yielded a significantly higher (p = 0.024) detection rate of EpCAM (+) CTCs. No statistically significant difference (p = 0.070) was found concerning the EpCAM status-based detection rate of CTCs by OQ+. (iii) CTC detection rates are independent of the primary tumors’ EpCAM expression. Conclusions. EpCAM (−) CTCs can not be detected by immunomagnetic EpCAM dependent enrichment methods. EpCAM independent enrichment technologies seem to be superior to detect the entire CTC population. Evaluation of CTCs as prognostic marker should compromise EpCAM (+) and (−) subpopulations.
Despite recent advances of new diagnostic and treatment strategies, breast cancer accounts for 500 000 deaths per year worldwide. Solid tumors of the breast can shed tumor cells into peripheral blood. These circulating tumor cells (CTCs) have a malignant potential and are able to form overt metastases in distant organs [Citation1]. The presence of CTCs can be observed also in neoadjuvantly treated breast cancer patients [Citation2]. In metastatic breast cancer (mBC) patients enrichment and detection of CTCs have an established clinical relevance in determining overall survival (OS) and progression-free survival (PFS) [Citation3]. This progress has been enabled by the development of CTC enrichment technologies using epithelial cell adhesion molecule (EpCAM) antibodies coupled to immunomagnetic beads [Citation4,Citation5].
EpCAM is an epithelial cell-specific surface antigen of 40 kDa. This glycoprotein is primarily localized within the intercellular boundaries of epithelia and mediates Ca2+-independent homophilic cell-cell adhesions [Citation6]. EpCAM is frequently overexpressed in different solid malignancies including colon, gastric, prostate, ovarian, lung, and breast cancer [Citation7,Citation8]. High EpCAM expression is found in 97% of colon cancers. In contrast, primary malignant breast cancer cells express high levels of EpCAM in only 41.7% [Citation7,Citation9]. Cimino et al. revealed that EpCAM expression on metastatic breast cancer tissue is consistently higher compared to the matched primary tissue [Citation10]. Furthermore, EpCAM expression seems to be down regulated in CTCs [Citation11]. So far, the coherence between EpCAM expression in primary breast cancer tissue and CTCs is a matter of speculation.
However, one preclinical in vitro study raised concerns regarding EpCAM dependent assays for detecting CTCs [Citation12]. A limitation of the anti-EpCAM antibody-based enrichment approach might be the heterogeneous EpCAM expression of diverse breast cancer subtypes. So far, this hypothesis has only been generated in breast cancer cell lines according to the five intrinsic breast cancer subtypes previously described [Citation12]. Although the reliability of immunomagnetic EpCAM-based CTC enrichment technologies has extensively been proven, CTCs can be detected in only 60% of mBC patients [Citation3,Citation4,Citation13]. One explanation for this low detection rate might be the EpCAM negativity of CTCs.
Besides EpCAM based CTC assays, enrichment strategies independent of the tumor cells’ EpCAM expression could be advantageous in isolating EpCAM negative CTCs. A new EpCAM independent density gradient centrifugation technology has recently been introduced by our study group [Citation14].
EpCAM based CTC enrichment technologies may miss EpCAM negative CTCs. To the best of our knowledge, the missing gap of detection between EpCAM positive (+) and EpCAM negative (−) CTCs has not been extensively studied in mBC patients. Thus, we addressed the question of the reliability of an EpCAM dependent assay to enrich CTCs in several breast cancer subtypes. Our study was prospectively designed to enrich and detect CTCs in vitro and in vivo depending on the respective EpCAM expression using two different CTC enrichment technologies. These two methods encompass one anti-EpCAM immunomagnetic enrichment technology, MACS HEA MicroBeads®, and one new EpCAM independent density centrifugation method, OncoQuick® plus. Furthermore, we tested the coherence between EpCAM expression on the primary tissue of mBC patients and the CTC detection rates of the corresponding patients.
Material and methods
REMARK guidelines and study design
All experimental and clinical data should be comprehensible and replicable. Thus, in presenting our results we adhere to the “REporting recommendations for tumor MARKer prognostic studies (REMARK guidelines)” [Citation15].
Cell culture and spiking experiments
Two luminal EpCAM (+) - MCF-7 and ZR-75-1- and one basal like EpCAM (−) - Hs 578T - cell lines (obtained from ATCC, Rockville, MD, USA) were used for flow cytometry and spiking experiments. The respective cell lines were chosen according to their EpCAM expression. Her/2neu status was not considered because it does not influence CTC enrichment and detection rates [Citation12]. All cell lines were cultured as monolayer in RPMI medium (GIBCO BRL, Gaithersburg, MD) supplemented with 10% fetal calf serum, 100 units/ml penicillin and 100 μg/ml streptomycin (GIBCO BRL, Gaithersburg, MD).
Cells were cultured to mid-log phase and harvested by trypsinization. Cells were resuspended in 1 ml PBS containing 2% FCS to abrogate trypsin activity. Blocking of unspecific binding was prevented with AB-serum (Biotest, Dreieich, Germany) for 15 min at room temperature.
To evaluate tumor cell recovery, we spiked a definite number of cells in 15 ml of peripheral blood of healthy donors. All spiking experiments were done in duplicates to test the reproducibility of the cell recovery rate.
Flow cytometry by lab-on-chip technology
For quantitative analysis of the EpCAM expression, MCF-7, ZR-75-1 and Hs578T cells were blocked with AB-serum (Biotest, Dreieich, Germany) for 15 min at room temperature to prevent unspecific binding and then were incubated with an antibody directed against EpCAM (Neomarkers, Fremont, CA, USA) in a concentration of 1 μg/ml for 30 min on ice. After washing, cells were labelled with Alexa Fluor 647 anti-mouse IgG antibody (Molecular Probes, Eugene, OR, USA) for 30 min on ice. Following another washing step cells were treated according to the instructions of the cell fluorescence assay and were analyzed with an Agilent Bioanalyzer 2100 system (Agilent, Palo Alto, CA, USA).
Blood collection and patients
We collected peripheral blood from 26 mBC patients. Patients were classified by breast cancer subtype, age, menopause status, type of surgery, histology, tumor size, lymph node status and tumor grade.
Peripheral blood from 20 healthy, female volunteers served as negative controls. Peripheral blood samples were collected in heparinized tubes (BD Vacutainer; Becton Dickinson, Heidelberg, Germany) and processed within 12 hours.
Study participants were informed of the investigational nature of this analysis and had given informed consent in accordance with institutional and federal guidelines. The study protocol was approved by the ethical committee of the participating institutions.
Tumor cell enrichment with anti-EpCAM antibodies and density gradient centrifugation
Tumor cell enrichment using anti-EpCAM antibodies, MACS HEA MicroBeads®, and density gradient centrifugation, OncoQuick® plus, were performed as previously described [Citation14]. For each cytometric enrichment procedure 15 ml of peripheral blood were used.
In brief, enrichment of CTCs was performed by direct immunomagnetic labelling of EpCAM positive cells by adding 200 μl FcR blocking and 200 μl HEA 125-microbeads (both obtained from Miltenyi Biotec Inc., Bergisch Gladbach, Germany). The microbead-cell suspension was incubated for 30 min on a shaker to enable CTCs to have maximum contact with MACS HEA MicroBeads®. The cell-microbead suspension was loaded in equal shares on MACS® Separation Columns (Miltenyi Biotec Inc., Bergisch Gladbach, Germany). The flow-through was discarded and in general not further analyzed. For each cell line mentioned above, one additional spiking experiment in duplicates was conducted to analyze the flow-through for EpCAM (−) and EpCAM (+) cells, respectively. The cell fraction in the columns was gently flushed out with the plunger delivered with the columns directly on the slides.
OncoQuick® plus (Greiner BioOne, Frickenhausen, Germany) is a complete prototype containing an optimized gradient separation medium that is commercially not yet available. OncoQuick® tubes were filled with 15 ml of 4°C cold peripheral blood. After centrifugation (20 min at 1600 × g with breaks off) interphase cells were poured into a precooled 50 ml tube (Greiner BioOne, Frickenhausen, Germany) pretreated with washing buffer overnight. Cells were washed twice with washing buffer at 200 × g for 10 min at 4°C. After the second washing step the cell pellet was resuspended in 1 ml of washing buffer.
To identify CTCs we used the murine monoclonal cytokeratin antibody A45-B/B3 (Micromet, Munich, Germany). Immunocytochemistry was performed as described in the manufacture's manual.
Additionally, 1 μl 4′6-diamid-ino-2-phenylindole (DAPI) was added to visualize cell nuclei. Slides were mounted with 250 μl Kaiser's glycerol gelatine (Merck, Darmstadt, Germany).
Immunohistochemical analyses and scoring
Formalin-fixed, paraffin embedded tissue from the primary breast lesions of the corresponding mBC patients was used for all immunostaining procedures. Four micrometer thick sections were cut and mounted to coated slides.
Immunostaining of estrogen receptor (ER), progesterone receptor (PR), Her/2neu, CK 5/6, EGFR and Ki-67 protein served as surrogate markers for intrinsic breast cancer molecular subtypes as previously described [Citation16,Citation17]. Immunostaining of ER, PR, Her/2neu and the Ki-67 protein were prospectively and routinely evaluated at the time of diagnosis of the primary tumor at the central pathology department, Medical University of Vienna, Austria. Immunostaining of CK 5/6, EGFR and EpCAM were retrospectively accomplished at the same institution.
Immunohistochemical (IHC) staining and scoring of ER, PR, Her/2neu receptor status and Ki-67 were accomplished as described elsewhere [Citation18,Citation19]. Tumors were considered positive for Her/2neu if immunostaining was scored as 3+ according to HercepTest criteria. Her/2neu receptor status was considered to be negative if membranous staining was 0 and 1+. Cases with a positive Her/2neu staining of 2+ on IHC analysis were further evaluated by fluorescent in situ hybridization (FISH) for amplification of the Her/2neu gene. CK 5/6 and EGFR (both obtained from Dako, Glostrup, Denmark) were used as immunohistochemical surrogate markers to define basal like breast cancers. Immunostaining and scoring were processed as described elsewhere [Citation16,Citation17,Citation20].
Patients were classified as luminal A (ER (+) and/ or PR (+) and not Her/2neu (−) or Ki-67high), luminal B (ER (+) and/ or PR (+) and Her/2neu (−) and/ or Ki-67 high), Her/2neu overexpressing (ER (+/−) PR (+/−) and Her/2neu positive) and basal like (ER (−), PR (−), Her/2neu (−), EGFR (+) and/ or CK 5/6 (+)).
Immunohistochemical staining of EpCAM (Progen, Heidelberg, Germany) was performed according to Momburg et al. [Citation21]. Primary breast cancer tissue was considered positive for EpCAM according to Spizzo and colleagues [Citation9].
EpCAM immunofluorescence staining of CTCs
Another 15 ml of peripheral blood were drawn from the consecutive metastatic breast cancer patients to evaluate EpCAM positivity. After enriching CTCs with the OncoQuick® plus procedure as described above, enriched cells were blocked with 5% goat serum (Dako, Glostrup, Denmark). Next, CTCs were incubated with a FITC conjugated mouse monoclonal antibody to EpCAM (clone VU-ID9; dilution 1:200; Abcam, Cambridge, UK) in a humified dark chamber for one hour and then washed three times with PBS for five minutes. Visualization of cell nuclei and mounting of slides were performed as described above.
Microscopic evaluation of CTCs
Spiked cells, CTCs and EpCAM expression of CTCs were enumerated and evaluated by two independent observers (RK, GP). Both observers were blinded to laboratory and clinical data. For the screening procedure an Olympus BX 50 immunofluorescence microscope (Olympus, Hamburg, Germany) was used. Inconclusive results were discussed and in cases of discrepant scoring, a final consensus was reached by using a double-headed microscope.
Cells were classified as CTCs when staining was positive for cytokeratin and when morphologic criteria were fulfilled according to recommended guidelines [Citation22].
MBC patients were considered to be EpCAM positive, if a clear membranous staining pattern was visible on fluorescently labeled tumor cells.
Statistical analysis
Descriptive analyses of recovery rates by the two different enrichment technologies using spiking experiments were achieved by means of notched box plots. Differences in tumor cell numbers between the two cytometric techniques were analyzed with Wilcoxon signed-rank test for paired non-normally distributed groups. In mBC patients, associations between CTCs and clinical parameters were assessed by χ2 or Fisher's exact test, where appropriate. Differences in CTC positivity in breast cancer patients between the two enrichment methods were assessed using McNemar test. All statistical calculations were performed using the SPSS 17.0 statistical software (Chicago, Illinois, USA). Statistical two-sided p-values <0.05 were considered significant.
Results
EpCAM expression in MCF-7, ZR-75-1 and Hs578T breast cancer cell lines
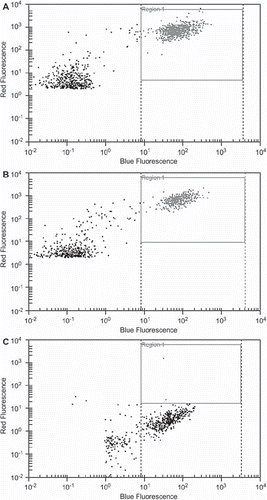
To evaluate the EpCAM expression of the three diverse cell lines, tumor cells were sorted according to their EpCAM expression. As shown in the luminal MCF-7 and ZR-75-1 cell lines showed a high (>90%) EpCAM expression. In the basal like Hs578T cells EpCAM expression was observed only in a small population (<5%). This difference was statistically significant (p < 0.001).
Recovery of luminal EpCAM (+) and basal like EpCAM (−) breast cancer cell lines by two enrichment methods
To evaluate the recovery rate of MACS HEA MicroBeads® and OncoQuick® plus a definite number of cells from the luminal EpCAM (+) MCF-7 and ZR-75-1 and from the basal like EpCAM (−) Hs578T cell lines, respectively, were spiked into 15 ml peripheral blood of female volunteers.
Mean number of spiked cells and median recovery rates of spiked tumor cells are described in .
Table I. Recovery rates of spiking experiments of the EpCAM (+) cell lines, MCF-7 and ZR-75-1, and the EpCAM (−) cell line, Hs 578T. To enrich tumor cells one EpCAM dependent assay, MACS HEA MicroBeads®, and one EpCAM independent assay, OncoQuick® plus, were used.
The EpCAM-based immunomagnetic method, MACS HEA MicroBeads®, recovered significantly more of luminal EpCAM (+) MCF-7 (p < 0.001) and ZR-75-1 (p < 0.001) tumor cells than basal like EpCAM (−) Hs587T tumor cells.
To evaluate the flow-through of MACS HEA MicroBeads® a definite number of cells from the luminal EpCAM (+) cell lines MCF-7 (60 and 57 tumor cells spiked, respectively), ZR-75-1 (36 and 31 tumor cells spiked, respectively), and from the basal like EpCAM (−) cell line Hs587T (29 and 29 tumor cells spiked, respectively), were spiked into peripheral blood; experiments were done in duplicates. Recovery rates for MCF-7, ZR-75-1 and Hs587T tumor cells were 73.50%, 79.10% and 3.45%, respectively.
Regarding the flow-through of the MCF-7 and ZR-75-1 cell lines 16.38% and 13.11%, respectively, of EpCAM (+) cells were rediscovered. Regarding the flow-through of the Hs587T cell line 82.76% of EpCAM (−) cells were rediscovered. In the mean a cell loss of 10.57% per spiking experiment was observed.
With the density gradient centrifugation system, OncoQuick® plus, no significant differences in the recovery rate were detected between the luminal EpCAM (+) MCF-7 (p = 0.796) or ZR-75-1 (p = 0.978) cell lines and basal like EpCAM (−) Hs587T tumor cells, respectively ().
Figure 2. Box plots demonstrating that basal like Hs 578T EpCAM (−) tumor cells are enriched to a significant lesser extent with the EpCAM dependent enrichment method, MACS HEA MicroBeads®, compared to luminal EpCAM (+) cell lines, MCF-7 and ZR-75-1. Vice versa, an EpCAM independent enrichment method, OncoQuick® plus, enriches EpCAM (−) and EpCAM (+) tumor cells without significant difference. MACS HEA MicroBeads® recovered significantly more EpCAM (+) cells compared to OncoQuick® plus. Vice versa, OncoQuick® plus recovered significantly more of EpCAM (−) Hs 578T tumor cells.
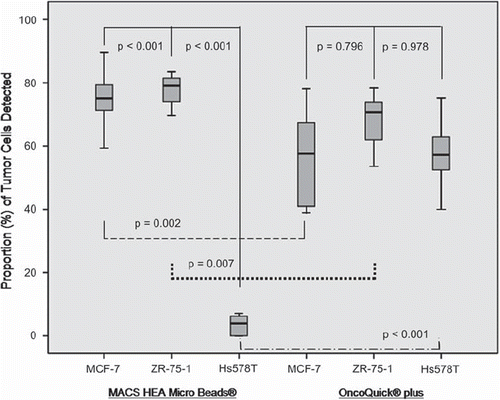
MACS HEA MicroBeads®, recovered significantly more luminal EpCAM (+) MCF-7 and ZR-75-1 tumor cells than OncoQuick® plus (p = 0.002 and p = 0.007, respectively). Vice versa, OncoQuick® plus recovered significantly more basal like EpCAM (−) Hs587T tumor cells (p < 0.001) than MACS HEA MicroBeads® ().
Assay specificity of MACS HEA MicroBeads® and OncoQuick® plus in healthy females
Specificity of the two enrichment technologies was tested by analyzing 15 ml aliquots of peripheral blood per method from 20 healthy, female volunteers. We did neither detect CTCs nor unspecific reactions with any of the two enrichment methods.
CTC detection with MACS HEA MicroBeads® and OncoQuick® plus in metastatic breast cancer patients
Peripheral blood samples from 26 chemotherapy naive mBC patients (median age 53 years, range 35–71) were prospectively collected between January and July 2009. No breast cancer patients were classified as luminal A. Further patients characteristics and the respective positivity rates of CTCs are presented in .
Table II. Detection rates of circulating tumor cells (CTCs) by two enrichment technologies according to the clinical pathological patient variables at the time of their diagnosis.
With MACS HEA MicroBeads® 42.3% of mBC patients had CTCs (median 0, range 0–3459). With OncoQuick® plus CTCs were spotted in 69.2% of patients (median 8, range 0–2895) resulting in a positivity rate of detected CTCs which did not differ significantly between MACS HEA MicroBeads® and OncoQuick® plus (p = 0.065) although the number of CTCs enumerated with OncoQuick® plus was significantly higher (p = 0.018).
CTC positivity rates by MACS HEA MicroBeads® and OncoQuick® plus were statistically not associated with basal like, luminal B and Her/2neu enriched breast cancer subtypes, respectively (). CTC counts according to the two CTC enrichment methods of luminal B, Her/2neu and basal like breast cancer patients, respectively, are presented in .
Table III. CTC detection rates according to the two CTC enrichment methods, MACS HEA MicroBeads® and OncoQuick® plus, of diverse breast cancer subtypes in 26 breast cancer patients.
Analysis of EpCAM expression
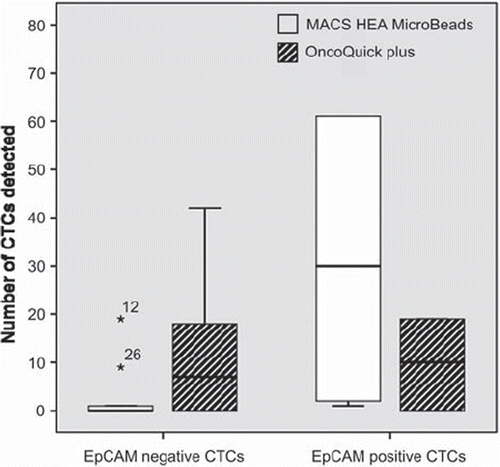
Overall, 38.5% of mBC patients had EpCAM (+) CTCs detected with immunofluorescence staining. The detection rate of EpCAM (+) CTCs with MACS HEA Micro Beads® was significantly higher compared to EpCAM (−) CTCs (p = 0.024) whereas no statistically significant association was found regarding the EpCAM status of CTCs detected with OncoQuick® plus (p = 0.070). The descriptive picture is presented in .
Figure 3. Box plots showing the detection rate of EpCAM (−) and EpCAM (+) CTCs enriched with MACS HEA Micro Beads® and OncoQuick® plus, respectively.
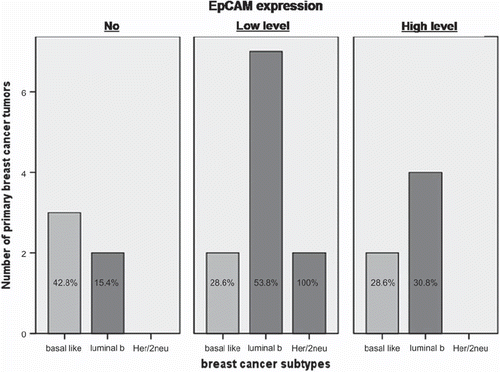
The EpCAM expression in primary breast cancer tissue of 22 patients (84.6%) was analyzed. High level of EpCAM expression was found in 27.3% of tumor samples, low level expression was found in 50.0% and 22.7% of tumor samples had no EpCAM expression at all. The descriptive picture is presented in .
Figure 4. No, low level and high level EpCAM expressions, respectively, according to primary breast cancer subtypes of 22 breast cancer patients.
From four (15.4%) breast cancer patients, biopsies of metastatic lesions including liver, lung and thyroid gland were available. EpCAM expression of the metastatic lesions was concordant to the respective EpCAM expression of the primary tumor. From the remaining 22 (84.6%) breast cancer patients no biopsies were obtained during the course of disease.
Neither with MACS HEA Micro Beads® nor with OncoQuick® plus detection of CTCs was significantly associated with EpCAM expression of the primary tumor (p = 0.209 and p = 0.179, respectively). In addition, no correlation (p = 0.656) was found between EpCAM positivity of CTCs and the corresponding EpCAM expression of the primary cancer tissue in the investigated cohort of mBC patients.
Discussion
In this prospectively planned analysis of CTC evaluation in chemotherapy naive metastatic breast cancer patients, detection of EpCAM (−) CTCs is highly depending on the enrichment method used. MACS HEA Micro Beads®, an EpCAM dependent immunomagnetic CTC enrichment method, detects significantly more EpCAM (+) than EpCAM (−) CTCs. On the other hand the EpCAM independent density centrifugation based method, OncoQuick® plus, enriches EpCAM (+) and EpCAM (−) CTCs without significant difference.
These results retrieve their analogy in our in vitro results. With MACS HEA Micro Beads® we recovered significantly more tumor cells from luminal EpCAM (+) cell lines. Otherwise OncoQuick® plus did not recover significantly more luminal EpCAM (+) compared to EpCAM (−) spiked tumor cells. Our spiking results of the diverse cell lines suggest that EpCAM based enrichment methods are unsuitable for detection of EpCAM (−) cell lines. Although OncoQuick® plus seems to be advantageous for capturing EpCAM (+) and EpCAM (−) tumor cells, this CTC enrichment technology has one major draw-back reflected by broad confidence intervals. Thus, the precision of this method is not as accurate as MACS HEA Micro Beads®.
However, the results from Sieuwerts et al. support our in vitro data. In that investigation the CellSearch® System, a semiautomatic immunomagnetic EpCAM based CTC enrichment and detection method, was used to evaluate the recovery rates of EpCAM (+) and EpCAM (−) cell lines spiked into peripheral blood. This preclinical study proposed that CTC enrichment of the diverse intrinsic breast cancer subtypes might be dependent on EpCAM expression [Citation12].
Of importance, the flow-through of spiking experiments was analyzed to examine the loss of EpCAM (+) and EpCAM (−) tumor cells. In the mean 10.57% of spiked tumor cells are lost during the enrichment and detection procedure. Regarding EpCAM (−) cells, 82.76% are found after staining with the A45B/B3 antibody in the flow-through. It might be an appealing idea to enrich and detect EpCAM (+) and EpCAM (−) cells by applying one method deposing on equal detection sensitivity for both EpCAM (+) and EpCAM (−) tumor cells. But, searching for CTCs in peripheral blood without enrichment is highly tedious. With the use of immunomagnetic enrichment the detection of the rare tumor cells (approximately 10e-7–10e-8) is substantially easier [Citation25], even at the cost of cell loss. Fluorescence-activated cell sorting (FACS) is an alternative enrichment technology tested preclinically to isolate and detect CTCs independent of EpCAM status [Citation26].
To the best of our knowledge the validation of in vitro data is lacking in breast cancer patients. Doing so we used previously described immunohistochemical surrogate markers to classify our patient cohort according to intrinsic breast cancer subtypes [Citation16,Citation17]. We did not detect a significant correlation between CTC positivity/count and basal like, luminal B or Her/2neu positive breast cancer subtypes, respectively.
However, EpCAM expression on specific cell lines skews the in vivo picture of metastatic breast cancer patients. Breast cancer cell lines represent the homogenous picture of primary cells with a distinct ER, PR, Her/2 and EpCAM status. In vivo, there is evidence that EpCAM expression of CTCs is down regulated in peripheral blood [Citation11], suggesting that a mixture of EpCAM (+) and EpCAM (−) tumor cells circulate in peripheral blood. Furthermore, primary breast cancer tissue has a heterogeneous EpCAM expression. About 10% of primary breast cancers have no EpCAM expression [Citation9]. To what extent EpCAM expression of primary breast cancer tissue has an impact on enrichment and detection of metastatic breast cancer patients has not been a matter of investigation so far. For all we know, we are the first study group pointing out a lack of a nexus between EpCAM expression of primary breast cancer tissue and the detection of CTCs of the matched metastatic breast cancer patients. These results are independent of the enrichment method used. From four breast cancer patients biopsies of metastatic lesions were available. Their EpCAM expression was concordant to the respective EpCAM expression of the primary tumor. It would be of interest to obtain more metastatic biopsies of metastatic lesions to correlate their EpCAM expression with the respective CTC detection rates to substantiate our preliminary results. But, this would imply an additional burden and invasive intervention for metastatic breast cancer patients.
The synopsis of our results and evidence from other study groups allow the conclusion that EpCAM based CTC enrichment technologies are strongly dependent on the CTC's EpCAM expression [Citation12,Citation23]. Immunomagnetic EpCAM-based methods may miss a subpopulation of CTCs. This might be one reason wherefore immunomagnetic EpCAM based enrichment technologies detect CTCs in only up to 60% [Citation5].
However, in our study population 69.2% of mBC patients had detectable CTCs with the EpCAM independent method, OncoQuick® plus. Our immunomagnetic EpCAM based enrichment technology, MACS HEA MicroBeads®, detected CTCs in 42.3% of the mBC patients. This CTC positivity rate of mBC patients with detectable CTCs did not differ significantly between the two methods. But, a significantly higher number of CTCs was enumerated with OncoQuick® plus. The reason for this important discrepancy lays in the fact that OncoQuick® plus enriches substantially more EpCAM (−) CTCs. Combining these findings we can deduce that irrespectively of breast cancer subtypes and EpCAM status both CTC enrichment methods seem to be suitable for CTC detection.
Nevertheless, our results depict a dangerous pitfall regarding CTC cut-off levels. Despite the use of the same EpCAM dependent assay different CTC thresholds have been established to identify mBC patients with a worse time to progression [Citation5,Citation13,Citation24].
CTC thresholds may be underestimated because of false negative results. These false negative results may be caused by EpCAM (−) CTCs that are not enriched with immunomagnetic based enrichment technologies. Further explanations for a lack of CTC detection may be on the one hand that no CTCs are present at all. On the other hand the pancytokeratin used for visualization of CTCs might be insufficient or CTCs undergo phenotypic changes, known as epithelial-mesenchymal transition [Citation25].
We would like to mention that our study is hypothesis generating. Since we were aware of the purely hypothesis generating character of our investigation, we felled it justified to include a restricted number of patients only. Despite of the small patient number, our data were found to be robust. Nevertheless, a prospective trial with a larger mBC patient cohort is warranted to allow validation.
In conclusion, the findings of our study support the clinical use of diverse EpCAM enrichment methods irrespectively of breast cancer subtypes. Neither EpCAM expression of the primary tumor tissue nor specific breast cancer subtypes of the primary tumor affect CTC enrichment rates. EpCAM (−) CTCs can not be detected by immunomagnetic EpCAM dependent enrichment methods. EpCAM independent enrichment technologies seem to be superior to detect the entire CTC population.
But in daily clinical routine, a simple cut-off number of a definite number of EpCAM (+) CTCs of a whole patient cohort seems not to be adequately representative for the individual mBC patient. Further investigations have to determine whether the accuracy for prognostication of mBC patients depends on the accurate detection of their EpCAM (+) and EpCAM (−) CTC subpopulations.
Acknowledgements
This investigation was supported by a research grant from the Austrian Research Promotion Agency (FFG)/CEADDP and was partly funded by Amgen and by a grant from the City of Vienna, Bürgermeisterfonds, Project number 08073. The study sponsors had no involvement in the study design, in the collection, analysis and interpretation of data, in the writing of the manuscript and in the decision to submit the manuscript for publication. We thank Michael Dahm, MD, (formerly Hexal Gentech Forschungs GmbH, Holzkirchen, Germany) for providing OncoQuick® plus. All authors declare that they have no potential conflicts of interest.
References
- Pretlow TG, Schwartz S, Giaconia JM, Wright AL, Grimm HA, Edgehouse NL, . Prostate cancer and other xenografts from cells in peripheral blood of patients. Cancer Res 2000;60:4033–6.
- Pierga JY, Bidard FC, Mathiot C, Brain E, Delaloge S, Giachetti S, . Circulating tumor cell detection predicts early metastatic relapse after neoadjuvant chemotherapy in large operable and locally advanced breast cancer in a phase II randomized trial. Clin Cancer Res 2008;14:7004–10.
- Cristofanilli M, Hayes DF, Budd GT, Ellis MJ, Stopeck A, Reuben JM, . Circulating tumor cells: A novel prognostic factor for newly diagnosed metastatic breast cancer. J Clin Oncol 2005;23:1420–30.
- Witzig TE, Bossy B, Kimlinger T, Roche PC, Ingle JN, Grant C, . Detection of circulating cytokeratin-positive cells in the blood of breast cancer patients using immunomagnetic enrichment and digital microscopy. Clin Cancer Res 2002; 8:1085–91.
- Riethdorf S, Fritsche H, Muller V, Rau T, Schindlbeck C, Rack B, . Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: A validation study of the CellSearch system. Clin Cancer Res 2007;13:920–8.
- Winter MJ, Nagtegaal ID, van Krieken JH, Litvinov SV. The epithelial cell adhesion molecule (Ep-CAM) as a morphoregulatory molecule is a tool in surgical pathology. Am J Pathol 2003;163:2139–48.
- Went P, Vasei M, Bubendorf L, Terracciano L, Tornillo L, Riede U, . Frequent high-level expression of the immunotherapeutic target Ep-CAM in colon, stomach, prostate and lung cancers. Br J Cancer 2006;94:128–35.
- Spizzo G, Went P, Dirnhofer S, Obrist P, Moch H, Baeuerle PA, . Overexpression of epithelial cell adhesion molecule (Ep-CAM) is an independent prognostic marker for reduced survival of patients with epithelial ovarian cancer. Gynecol Oncol 2006;103:483–8.
- Spizzo G, Went P, Dirnhofer S, Obrist P, Simon R, Spichtin H, . High Ep-CAM expression is associated with poor prognosis in node-positive breast cancer. Breast Cancer Res Treat 2004;86:207–13.
- Cimino A, Halushka M, Illei P, Wu X, Sukumar S, Argani P. Epithelial cell adhesion molecule (EpCAM) is overexpressed in breast cancer metastases. Breast Cancer Res Treat 2009.
- Rao CG, Chianese D, Doyle GV, Miller MC, Russell T, . Expression of epithelial cell adhesion molecule in carcinoma cells present in blood and primary and metastatic tumors. Int J Oncol 2005;27:49–57.
- Sieuwerts AM, Kraan J, Bolt J, van der Spoel P, Elstrodt F, Schutte M, . Anti-epithelial cell adhesion molecule antibodies and the detection of circulating normal-like breast tumor cells. J Natl Cancer Inst 2009;101:61–6.
- Hayes DF, Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Miller MC, . Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res 2006;12:4218–24.
- Königsberg R, Gneist M, Jahn-Kuch D, Pfeiler G, Hager G, Hudec M, . Circulating tumor cells in metastatic colorectal cancer: Efficacy and feasibility of different enrichment methods. Cancer Lett 2010;293:117–23.
- McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. REporting recommendations for tumor MARKer prognostic studies (REMARK). Breast Cancer Res Treat 2006;100:229–35.
- Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z, . Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res 2004;10:5367–74.
- Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, . Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst 2009; 101:736–50.
- Aft R, Naughton M, Trinkaus K, Watson M, Ylagan L, Chavez-MacGregor M, . Effect of zoledronic acid on disseminated tumour cells in women with locally advanced breast cancer: An open label, randomised, phase 2 trial. Lancet Oncol 2010;11:421–8.
- Kubista E, Planellas Gomez JV, Dowsett M, Foidart JM, Pohlodek K, . Effect of tibolone on breast cancer cell proliferation in postmenopausal ER+ patients: Results from STEM trial. Clin Cancer Res 2007;13:4185–90.
- Pohl G, Rudas M, Taucher S, Stranzl T, Steger GG, Jakesz R, . Expression of cell cycle regulatory proteins in breast carcinomas before and after preoperative chemotherapy. Breast Cancer Res Treat 2003;78:97–103.
- Momburg F, Moldenhauer G, Hammerling GJ, Moller P. Immunohistochemical study of the expression of a Mr 34,000 human epithelium-specific surface glycoprotein in normal and malignant tissues. Cancer Res 1987;47: 2883–91.
- Borgen E, Beiske K, Trachsel S, Nesland JM, Kvalheim G, Herstad TK, . Immunocytochemical detection of isolated epithelial cells in bone marrow: Non-specific staining and contribution by plasma cells directly reactive to alkaline phosphatase. J Pathol 1998;185:427–34.
- Mostert B, Kraan J, Bolt-de Vries J, van der Spoel P, Sieuwerts AM, Schutte M, . Detection of circulating tumor cells in breast cancer may improve through enrichment with anti-CD146. Breast Cancer Res Treat 2010.
- Bidard FC, Mathiot C, Degeorges A, Etienne-Grimaldi MC, Delva R, Pivot X, . Clinical value of circulating endothelial cells and circulating tumor cells in metastatic breast cancer patients treated first line with bevacizumab and chemotherapy. Ann Oncol 2010.
- Aktas B, Tewes M, Fehm T, Hauch S, Kimmig R, Kasimir-Bauer S. Stem cell and epithelial-mesenchymal transition markers are frequently overexpressed in circulating tumor cells of metastatic breast cancer patients. Breast Cancer Res 2009;11:R46.
- Eifler RL, Lind J, Falkenhagen D, Weber V, Fischer MB, Zeillinger R. Enrichment of circulating tumor cells from a large blood volume using leukapheresis and elutriation: Proof of concept. Cytometry B Clin Cytom 2010.