Abstract
Background. Intravesical administration is an important treatment against superficial bladder cancer and CD40L is essential for the protective anti-tumor immunity. In situ gene therapy with CD40L was demonstrated to successfully inhibit tumor cell growth in the orthotopic mouse model of bladder cancer. In the present study, we prepared streptavidin (SA)-tagged sCD40L and developed a novel immunotherapy for superficial bladder cancer based on the strong interaction between streptavidin and biotin. Material and methods. The SA-sCD40L fusion protein was expressed in E. coli and purified on the Ni-NTA column. After refolding with dialysis, the bi-function of the fusion protein was determined by flow cytometric analysis for streptaidin-mediated surface modification of MB49 bladder cancer cells and a mouse B cell CD40L-dependent proliferation assay. The mouse orthotopic model of MB49 superficial bladder cancer was used to evaluate the efficacy of SA-sCD40L immunotherapy. Results. The SA-sCD40L fusion protein exhibited both full biotin-binding property and CD40L bioactivity. After intravesical instillation, the SA-sCD40L bi-functional fusion protein was durably immobilized on the biotinylated mucosal surface of bladder wall for up to four days. The SA-sCD40L treatment significantly prolonged the survival of MB49 tumor-bearing mice and cured 50% of mice with MB49 superficial bladder cancer without significant adverse effects. In addition, more tumor-infiltrating CD4+or CD8+ T cells were observed in SA-sCD40L–treated group. Conclusion. Intravesical immobilization of SA-sCD40L elicited a strong and long-lasting immunity against the MB49 bladder cancer.
Bladder cancer is a common urological malignancy and more than 70% of new cases are diagnosed as superficial or non-muscle invasive cancer. Intravesical administration is an important measurement for superficial bladder cancer. Agents for intravesical therapy include biologics such as BCG and cytokine, and chemotherapeutics such as mitomycin C, epirubicin and doxorubicin. Intravesical instillation with BCG after transurethral resection is by far the most effective therapy for superficial bladder cancer. However, 30% of the patients develop resistance to BCG and 20–40% of the patients eventually relapse [Citation1,Citation2].
CD40/CD40L is central to the development of anti-tumor immunity. CD40L gene therapy has been demonstrated an efficient strategy for bladder cancer [Citation3]. Transgenic expression of CD40L in a small proportion of neuroblastoma cells was enough to generate a long-lasting systemic anti-tumor immune response in mice [Citation4]. Gene therapy by use of recombinant adenovirus expressing CD40L (AdCD40L) has been proven effective in syngeneic tumor models of colorectal carcinoma, lung carcinoma and melanoma [Citation5]. Recently, it was reported that vaccination with CD40L-expressing TRAMP-C2 cells induced anti-tumor immunity and peritumoral AdCD40L injections induced tumor growth suppression [Citation6]. It was reported that CD40 activation could have a direct anti-tumor effect on several tumors [Citation7]. In addition, CD40L can promote maturation and activation of DC so as to enhance the anti-tumor immunity [Citation8]. CD40L also can promote the CD40+tumor cell apoptosis directly [Citation9–12]. CD40L belongs to the TNF superfamily as a type II transmembrane protein, and is expressed mainly by activated T cells, activated B cells and platelets [Citation13,Citation14]. Soluble CD40L (sCD40L) has been reported to have similar activities to transmembrane form, and incorporation of an Isoleucine Zipper Motif enhances the biological activity of sCD40L [Citation15,Citation16].
Streptavidin (SA) is a streptomyces avidinii-derived homo-tetrameric protein, which binds to biotin in an extremely high affinity with the Kd value of 10−15 M, 103 to 106 folds higher than that for typical antigen-antibody interaction [Citation17,Citation18].
In the present study, we generated a SA-sCD40L bi-functional fusion protein, i.e., biotin-binding property and sCD40L activity, and evaluated its antitumor activity in mouse orthotopic model of MB49 superficial bladder cancer. It was reported that instillation of MB49 tumor cells into unmodified bladders did not result in tumor take, and poly-L-lysine or ethanol pretreatment was proven to be successful to inoculate MB49 cells into the bladder [Citation19]. In this paper, in order to prepare the bladder for tumor implantation, a brief acid pretreatment was performed. Our results showed that immobilization of SA-sCD40L on the biotinylated mucosal surface of bladder wall elicited a strong and long-lasting immunity against the MB49 bladder cancer.
Material and methods
Animals and cell culture
C57BL/6 female mice at approximately eight weeks old were purchased from the certified experimental animal center at Southern Medical University (Guangzhou, China). All animal studies were carried out in accordance with the university guidelines for experimental animals. The MB49 cell line, a gift from Dr I. C. Summerhayes in Lahey Clinic (Burlington, MA, USA), was cultured in DMEM supplemented with 10% FBS, 1% penicillin/streptomycin and 2 mM L-glutamine at 5% CO2, 37°C.
Construction of SA-sCD40L expressing plasmid
Total RNA was isolated from ConA-activated murine spleen cells with Trizol (Invitrogen, Carlsbad, CA, USA). The cDNA encoding murine sCD40L (amino acids 112-260) was amplified by RT-PCR, using the following primer pair: 5′GGAATTCATGCAAAGAGGTGATGAGGA3′(27nt), which included an EcoR I site, and 5′CCTCGAGTCAGAGTTTGAGTAAGCCAA3′ (27nt), which included a Xho I site and a stop codon. The cDNA encoding mature SA was obtained by PCR with the genomic DNA of S. avidinii (ATCC, Manassas, VA, USA) as a template and the following primer pair: 5′GGAATTCCATATGCATCATCACCATCACCATGAGGCCGGCATCACCGGCACCTGG3′(55nt), which included a Nde I site, and 5′GGAATTCGCCGGATCCGC CCCCGCCGCTGCCTCCGCCCCCGCTGCCCCCGCTCGTCTGCTGAACGGCGTCGAGCGGGTTGCC3′ (82nt), which included an EcoR I site. The two PCR products were cloned into Nde I and Xho I sites of pET24a vector (Novagen, Madison, CA, USA). The SA-CD40L fusion protein was designed to contain single 6-His tag at its N-terminal and the glycine/serine-rich flexible linker with the following DNA sequence: ACG AGC GGG GGC AGC GGG GGC GGA GGC AGC GGC GGG GGC GGA TCC GGC GAA TTC.
Expression of SA-sCD40L fusion protein
Five hundred milliliters of LB medium containing 50 μg/ml kanamycin was inoculated with 5 ml of overnight culture of E. coli containing the recombinant construct and incubated at 37°C until OD600 of the culture reached 0.6. The expression of SA-sCD40L fusion protein was induced by the addition of IPTG to a final concentration of 1 mM. The culture was incubated for additional four hours and the cells were harvested by centrifugation at 5000 g for 15 minutes at 4°C. The pellet was suspended in 30 ml of cell lysis buffer by gentle vortexing for 20 minutes and then disrupted by sonication. The expression of SA-sCD40L fusion protein was analyzed by SDS-PAGE.
Purification of SA-sCD40L fusion protein
SA-sCD40L fusion protein was expressed as an inclusion body. The cell lysate was subjected to centrifugation at 20 000 g for 30 minutes at 4°C, and the supernatant was decanted. The pellet was collected and washed consecutively for 30 minutes on a rocking platform at 4°C with the following solutions: A (20 mM Tris-HCl, 2 mM EDTA, pH 8.0), B (20 mM Tris-HCl, 10 mM EDTA, 2 mM mercaptoethanol, 0.1% Triton X-100, pH 8.0), C (20 mM Tris-HCl, 2 M Urea, 2 mM EDTA, pH 8.0), D(20 mM Tris-HCl, 50% isopropanol, 2 mM EDTA, pH 8.0) and E (20 mM Tris-HCl, pH 8.0). The washed inclusion body was dissolved in the column buffer (50 mM sodium phosphate, 8 M urea, 10 mM mercaptoethanol, 10 mM imidazole, pH 8.0). The supernatant was filtered with 0.45 μm membrane and applied to the Ni-NTA column (Qiagen, Valencia, CA, USA) according to the manufacturer's instruction. The column was equilibrated with 200 ml of the buffer containing 30 mM imidazole, and SA-sCD40L fusion protein was eluted with 100 mM imidazole.
Refolding of SA-sCD40L fusion protein
The SA-sCD40L fusion protein was refolded by the gradual removal of urea. Briefly, the purified fusion protein SA-sCD40L was adjusted to 0.1 mg/ml and dialyzed successively against 20 volume of each refolding buffer as follows with slow stirring at 4°C for 12 hours: A (20 mM NaHCO3, 4 M Urea, 0.3 M L-Arginine 1 mM GSH, 0.1 mM GSSG, 1 mM EDTA, pH 10.0), B (20 mM NaHCO3, 2 M Urea, 0.3 M L-Arginine 1 mM GSH, 0.1 mM GSSG, 1 mM EDTA, pH 10.0), C (20 mM NaHCO3, 1 M Urea, 1 mM GSH, 0.1 mM GSSG, 1 mM EDTA, pH 10.0). After centrifugation, the refolded SA-sCD40L fusion protein was further purified through the 2-iminobiotin agarose column (Sigma, St. Louis, MO, USA). The bound SA-sCD40L fusion protein was eluted with 50 mM NaAc, 150 mM NaCl, pH 4.0, and then adjusted to pH 8.0 with 500 mM (NH4)2CO3, 5.0 M NaCl, pH 11, followed by Detoxi-Gel Endotoxin Removing Gel (Pierce, Rockford, IL, USA) column to remove bacterial endotoxin contaminants. The filtered preparation of SA-sCD40L fusion protein was stored in −20°C. The prepared SA-sCD40L fusion protein was quantitatively measured by use of mouse sCD40L ELISA Kit (Bender MedSystems, Vienna, Austria).
SDS-PAGE and Western blotting
SA-sCD40L fusion protein was analyzed by SDS-PAGE and stained with Coomassie brilliant blue R-250. The refolded SA-sCD40L fusion protein was separated by SDS-PAGE and transferred onto a polyvinylidene difluoride membrane in a transfer apparatus. The blot membrane was immersed in phosphate buffered saline with 0.5% Tween-20 (PBST) supplemented with 5% skim milk for one hour at 37°C and subsequently incubated in hamster anti-mouse CD40L antibody (BD Biosciences, San Diego, CA, USA) at 1:500 dilution in the blocking buffer for one hour at 37°C. After washing three times with PBST, the membrane was incubated in anti-hamster IgG conjugated horseradish peroxidase at 1:4000 for one hour at 37°C. After extensive washing, the membrane was developed with DAB (3, 3′-diaminobenzidine) display liquid kit (Boshide Corporation, Wuhan, China).
Bioactive assays in vitro for SA-sCD40L bifunctional fusion protein
The biological activity of SA-sCD40L fusion protein was determined by flow cytometric analysis and a mouse B cell proliferation assay. To examine the biotin-binding property of the fusion protein, 5 × 106 MB49 cells were incubated in 1xPBS containing 1.0 mg/ml EZ-Link NHS-PEO4-Biotin(Pierce) for 30 minutes at room temperature. After washing three times with 1xPBS + 100 mM glycine, the cell suspension was incubated in 0.1 mg/ml SA-sCD40L for 60 minutes at room temperature. After extensive washing with 1xPBS, the modified cells were incubated with hamster anti-mouse CD40L antibody for 30 minutes at 37°C, and then detected with FITC-conjugated anti-hamster IgG to determine the cell-surface modification efficiency of SA-CD40L fusion protein with a flow cytometer (Becton Dickinson, San Jose, CA, USA).
The CD40L biological activity of the fusion protein was assessed by a mouse B cell proliferation assay in the presence of recombinant IL-4 as a co-stimulus. Briefly, mouse B cells were collected from splenocytes by density gradient centrifugation with Ficoll paque and nylons wool column. Indicated concentrations of SA-sCD40L were incubated with 1 × 105 B cells with recombinant IL-4 (10 ng/ml) per well in 96-well plates. After incubation for 72 hours at 37°C in 5% CO2, 20 μl of 5 mg/ml 3- (4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) solution was added to each well and incubated for four hours. The plate was centrifuged at 2000 g for 10 minutes, and 100 μl of dimethylsulfoxide was added to each pellet to dissolve the formazan dye for 10 minutes. Absorbance was measured at a test wavelength of 570 nm and a reference wavelength of 630 nm with a microplate reader.
Immunotherapy for superficial bladder cancer with SA-sCD40L fusion protein
The mouse orthotopic model of MB49 superficial bladder cancer was used to evaluate the efficacy of SA-sCD40L immunotherapy. Briefly, C57BL/6 female mice were anaesthetized with sodium pentobarbital intraperitoneally (68 mg/kg body weight), and then catheterized with 24-gauge Insyte IV using mineral oil as lubricant. The bladder was instilled with 100 μl of 0.1 mol/L HCl for 15 s and succeeding alkaline neutralization for 5 s. After washing three times with 1xPBS, 100 μl of MB49 cells (1 × 106) were instilled and incubated in the bladder for one hour. One day after MB49 tumor implantation, 100 μl of 1 mg/ml NHS-PEO4-biotin was incubated in the bladder for 30 minutes and washed with 1xPBS three times, followed by intravesical administration of 100 μl of 1xPBS, 0.15 mg/ml sCD40L, SA-GFP or SA-sCD40L to incubate for one hour. The treatment was performed twice a week for three weeks. The mice were monitored for health status (body temperature, body weight and food consumption), hematuria and palpable tumor.
Tumor-specific cytotoxicity assay
The splenocytes, collected from each group seven days after last intravesical therapy, were incubated with 20 U/ml of recombinant IL-2 and irradiated MB49 tumor cells for five days at 5% CO2. Effector cells were harvested and adjusted to the concentration of 5 × 106/ml. 1 × 104 MB49 cells were added per well as target cells, and varying number of effector cells were added in triplicate to the target cells at the indicated rations. After incubation for six hours, the plate was centrifuged at 250 g for four minutes, and 100 μl of culture supernatant was collected to measure LDH activity. The mean percentage cytotoxicity for each effector to target cell ratio was computed by the following formula: % cytotoxicity = (experimental - effector spontaneous - target spontaneous) / (target maximum - target spontaneous) × 100%.
Immunohistochemical analysis for tumor-infiltrating lymphocytes
To detect the persistence of SA-sCD40L on the biotinylated mucosal surface of bladder wall, the SA-sCD40L-treated mice were killed to obtain their bladders for frozen section on day 2, 4 and 6 after intravesical instillation. Sections of 10 μm were cut and stained with hamster anti-mouse CD40L antibody. After detection with anti-hamster IgG-HRP and succeeding development by DAB display liquid kit, sections were counterstained with hematoxylin.
To investigate the profile of tumor-infiltrating lymphocytes, three mouse bladders from each group were removed seven days after last intravesical therapy and then were snap-frozen. Sections of 10 μm were cut and stained with rat anti-mouse CD4+ or CD8+antibody. After visualization with anti-rat IgG-HRP detection kit, sections were counterstained with hematoxylin. For each slide ten consecutive adjacent digital images of fields located at the infiltrative edge of the tumor were acquired, starting at a random point. The positively-stained cells were counted and presented as median ± SD.
Statistical analysis
The results were representative of at least three independent experiments. The bioactivity assay was statistically analyzed with an unpaired t-test. Survival curve was plotted using the Kaplan-Meier method, and the difference in survival between the different groups was compared by the long-rank test. P < 0.05 was considered statistically significant. The statistical analyses were performed with SPSS 17.0.
Results
Preparation of SA-sCD40L bi-functional fusion protein
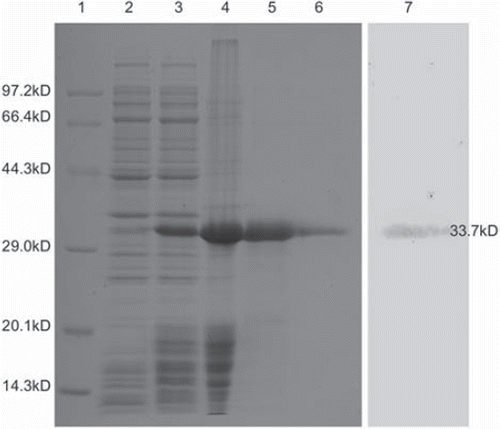
The DNA sequence of SA-sCD40L fusion gene was confirmed by DNA sequencing using T7 promoter and T7 terminator primers. After IPTG induction, SA-sCD40L fusion protein was expressed at 10% of total mass of bacterial protein mostly as inclusion body. After extracting, SA-sCD40L fusion protein was present at 60% in inclusion body. The purity of SA-sCD40L fusion protein was 90% after Ni-NTA affinity column. The purified SA-sCD40L fusion protein was refolded by gradual removal of urea (), followed by the 2-iminobiotin agarose column to reach the purity of 95%. The SA-sCD40L bi-functional fusion protein in the preparations showed no significant degradation after 6-month storage at −20°C.
Figure 1. Analysis of SA-sCD40L fusion protein by 12% SDS-PAGE and Western blotting. Lane 1: Protein molecular weight marker; Lane 2: Un-induced control; Lane 3: IPTG-induced expression; Lane 4: Inclusion body; Lane 5: After purification through Ni-NTA column; Lane 6: Refolded SA-sCD40L; Lane 7: Identification with hamster anti-mouse CD40L antibody.
Bi-functional activity of SA-sCD40L
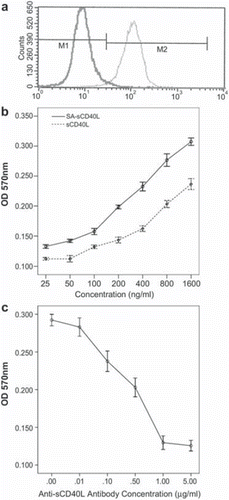
The refolded SA-sCD40L fusion protein exhibited both full biotin-binding property and sCD40L bioactivity. Flow cytometry showed that 98% biotinylated cells were modified with SA-sCD40L fusion protein (). The fusion protein also could stimulate B cells to proliferate in the presence of IL-4 as a costimulus in a dose-dependent manner. The specific activity of SA-sCD40L was increased compared with that of recombinant mouse sCD40L (PeproTech Inc, Rocky Hill, NJ, USA) as a control (). Furthermore, proliferation through induction of SA-sCD40L fusion protein was inhibited when anti-sCD40L antibody was added into the culture ().
Figure 2. Bi-functional activity of SA-sCD40L. The display of SA-sCD40L on the surface of biotinylated MB49 cells was examined by flow cytometric analysis with unbiotinylated MB49 cells as a control (Figure 2a). Indicated concentrations of SAsCD40L were incubated with 1 × 105 B cells with recombinant IL-4 (10ng/ml) per well in 96-well plates. After incubation for 72 hours, cell proliferation was measured by MTT assay (Figure 2b). Furthermore, proliferation through induction of SA-sCD40L fusion protein was inhibited when anti-sCD40L antibody was added into the culture (Figure 2c).
Immunotherapy with SA-sCD40L for superficial bladder cancer
The mouse orthotopic model of MB49 superficial bladder cancer was used to evaluate the efficacy of SA-sCD40L immunotherapy. One day after MB49 tumor cell implantation, intravesical administration with SA-sCD40L was performed twice a week for three weeks. No adverse effects were observed in the SA-CD40L-treated mice, such as fever, lassitude, weight loss and reduction of food consumption. On day 60 after the implantation of MB49 cancer cells, all of PBS- and sCD40L- treated mice died and 80% of SA-GFP-treated mice died from tumor burden. In contrast, 50% of mice in SA-sCD40L-treated group were tumor-free (). Moreover, the MB49 tumor-specific cytotoxicity from the tumor-free mice in SA-CD40L group was 11%, 25% and 35% at the effector to target ratios of 1:1, 25:1 and 50:1, respectively. But the corresponding cytotoxicity was 4%, 7% and 12% in sCD40L group, or 6%, 9%, 13% in SA-GFP group, or 5%, 7%, 11% in PBS group. The tumor-specific cytotoxicity in the SA-CD40L group was significantly higher than that in the control groups (p < 0.01). Immunohistochemical analysis showed that SA-sCD40L could be durably immobilized on the biotinylated mucosal surface of bladder wall for four days. The nascent urothelium, which efficiently replaced the acid-damaged urothelium after the instillation, was absent from biotinylation so as to make the major of mucosal surface not be stained (). More CD4+ T and CD8+ T cells infiltrated into the tumor sites of SA-CD40L-treated mice than those of other groups (, ).
Figure 3. Persistence of SA-sCD40L on the biotinylated mucosal surface of bladder wall. The SA-sCD40L-treated mice were killed to obtain their bladders on day 2, 4 and 6 day after intravesical instillation. Sections of 10 μm were cut and stained anti-mouse sCD40L antibody. The unbiotinylated bladder was used as a negative control.
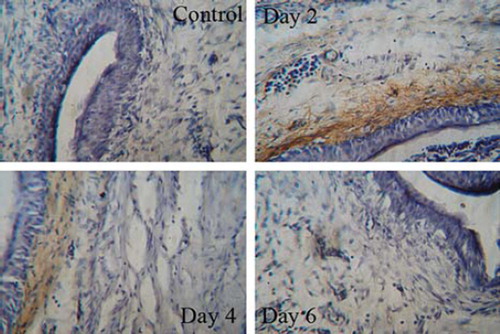
Figure 4. Immunohistochemical analysis for tumor-infiltrating lymphocytes. Bladders were removed from mice 7 days after last intravesical therapy and snap-frozen. Sections of 10 μm were cut and stained with rat-anti-mouse CD4+ or CD8+ antibody. After detection with anti-rat IgG-HRP and development by DAB display liquid kit, sections were counterstained with hematoxylin.
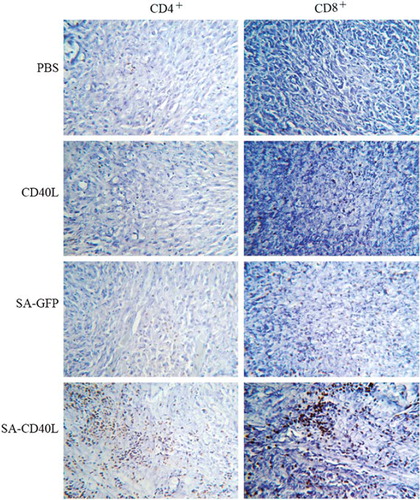
Figure 5. The therapeutic effect of SA-sCD40L fusion protein on the mouse orthotopic model of MB49 superficial bladder cancer. Mice were instilled with PBS, sCD40L, SA-GFP or SA-sCD40L one day after MB49 cells implantation. Each group had 10 mice. The survival curves were significantly different between the SA-CD40L group and the sCD40L group (p < 0.001), and between the SA-CD40L group and SA-GFP-treated group (p = 0.041).
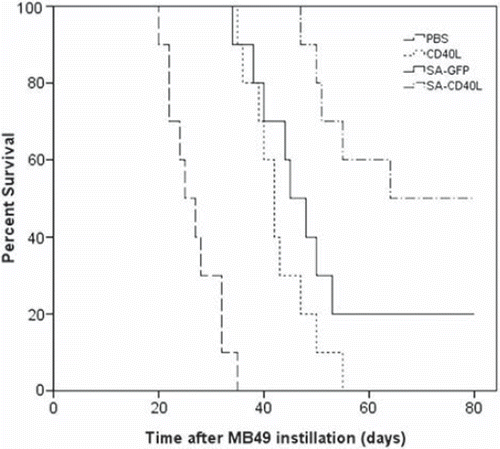
Table I. Infiltration of CD4+ T and CD8+ T cells into the tumor tissue.
Taken together, our study demonstrated that intravesical immobilization of SA-sCD40L elicited a strong and long-lasting immunity against the superficial bladder cancer.
Discussion
In the present study, we prepared SA-sCD40L bi-functional fusion protein, which was expressed in E. coli. The refolded SA-sCD40L fusion protein exhibited both full biotin-binding property and CD40L bioactivity. In addition, we prepared recombinant sCD40L protein, which was also expressed in E. coli. The sCD40L protein was easily precipitated out in the refolding buffer no matter how the protein concentration, pH or the urea gradient was adjusted. Thus, incorporation of streptavdin may improve the refolding efficiency of sCD40L.
Based on the specific and strong interaction between streptavidin and biotin, we developed a novel intravesical immunotherapy for superficial bladder cancer with SA-sCD40L bi-functional fusion protein. Our results showed that the SA-sCD40L fusion protein was efficiently and durably immobilized on the biotinylated mucosal surface of bladder wall. Furthermore, the treatment was demonstrated to significantly decrease the incidence of the implanted MB49 bladder cancer.
Previously, we reported that immobilization of SA-GM-CSF generated effective anti-tumor immunity against MB49 bladder cancer so that 67.5% cure rate was achieved [Citation20]. Taken into account of the microenvironment of tumor growth and the complexity of antitumor immunity, it is theoretically speculated that different immunostimulators such as cytokines, co-stimulators and chemokines may induce various effects individually and thus the efficacy should be improved remarkably in a combinatory way among the SA-tagged-bioactive cytokines or/and co-stimulators or/and chemokines, which is currently under our investigation.
In addition, the treatment with SA-GFP fusion protein increased the animal survival. This may be due to the strong immunogenicity of streptavidin in mouse. Streptavidin is a bacterial protein, which could induce humoral immune response. GFP is also an exogenous protein for mouse. Thus, SA-GFP, as an exogenous fusion protein, could stimulate immune response in mice so as to enhance their immunocompetence. However, a significant improvement of the immunotherapy was observed in the SA-sCD40L group in comparison with the SA-GFP group, indicating that the majority of the therapeutic efficacy resulted from the immunostimulation with immobilized sCD40L.
Therefore, intravesical immobilization of SA-sCD40L may represent a promising immunotherapy for superficial bladder cancer after transurethral resection.
Acknowledgements
This work was supported in part by grants from Chinese National 863 plan (2006AA02Z4C4), the National Natural Science Foundation of China (30928023, 30901822 and 30971516), Zhejiang Provincial Major Research Program (2007C13020, 2008C14082 and 2010C13007), the Natural Science Foundation of Zhejiang Province (R2080407 and Y2100925), Zhejiang Provincial Program for the Cultivation of High-level Innovative Health Talents, and Wenzhou Municipal Research Program (G20090142). Science and Technology Innovation Program of Activities for Students in Zhejiang Province (Emerging Artists Talents Scheme) (2009R413042).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Belldegrun AS, Franklin JR, O'Donnell MA, Gomella LG, Klein E, Neri R, . Superficial bladder cancer: The role of interferon-alpha. J Urol 1998;159:1793–801.
- Nawrocki S, Skacel T, Skoneczna I. Pharmacotherapy of bladder cancer – practice and prospects. Expert Opin Pharmacother 2002;3:671–9.
- Loskog AS, Fransson ME, Totterman TT. AdCD40L gene therapy counteracts T regulatory cells and cures aggressive tumors in an orthotopic bladder cancer model. Clin Cancer Res 2005;11:8816–21.
- Grossmann ME, Brown MP, Brenner MK. Antitumor responses induced by transgenic expression of CD40 ligand. Hum Gene Ther 1997;8:1935–43.
- Kikuchi T, Crystal RG. Anti-tumor immunity induced by in vivo adenovirus vector-mediated expression of CD40 ligand in tumor cells. Hum Gene Ther 1999;10: 1375–87.
- Dzojic H, Loskog A, Totterman TH, Essand M. Adenovirus-mediated CD40 ligand therapy induces tumor cell apoptosis and systemic immunity in the TRAMP-C2 mouse prostate cancer model. Prostate 2006;66:831–8.
- Elgueta R, Benson MJ, de Vries VC, Wasiuk A, Guo Y, Noelle RJ. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev 2009;229:152–72.
- van Mierlo GJ, den Boer AT, Medema JP, van der Voort EI, Fransen MF, Offringa R, . CD40 stimulation leads to effective therapy of CD40(-) tumors through induction of strong systemic cytotoxic T lymphocyte immunity. Proc Natl Acad Sci USA 2002;99:5561–6.
- Young LS, Eliopoulos AG, Gallagher NJ, Dawson CW. CD40 and epithelial cells: Across the great divide. Immunol Today 1998;19:502–6.
- Hess S, Engelmann H. A novel function of CD40: Induction of cell death in transformed cells. J Exp Med 1996;183: 159–67.
- Tong AW, Papayoti MH, Netto G, Armstrong DT, Ordonez G, Lawson JM, . Growth-inhibitory effects of CD40 ligand (CD154) and its endogenous expression in human breast cancer. Clin Cancer Res 2001;7:691–703.
- Yamada M, Shiroko T, Kawaguchi Y, Sugiyama Y, Egilmez NK, Chen FA, . CD40-CD40 ligand (CD154) engagement is required but not sufficient for modulating MHC class I, ICAM-1 and Fas expression and proliferation of human non-small cell lung tumors. Int J Cancer 2001;92:589–99.
- Carbone E, Ruggiero G, Terrazzano G, Palomba C, Manzo C, Fontana S, . A new mechanism of NK cell cytotoxicity activation: The CD40-CD40 ligand interaction. J Exp Med 1997;185:2053–60.
- Elgueta R, Benson MJ, de Vries VC, Wasiuk A, Guo Y, Noelle RJ. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev 2009;229:152–72.
- Mazzei GJ, Edgerton MD, Losberger C, Lecoanet-Henchoz S, Graber P, Durandy A, . Recombinant soluble trimeric CD40 ligand is biologically active. J Biol Chem 1995;270: 7025–8.
- Morris AE, Jr Remmele RL, Klinke R, Macduff BM, Fanslow WC, Armitage RJ. Incorporation of an isoleucine zipper motif enhances the biological activity of soluble CD40L (CD154). J Biol Chem 1999;274:418–23.
- Sano T, Cantor CR. Streptavidin-containing chimeric proteins: Design and production. Methods Enzymol 2000;326: 305–11.
- Gao J, Huang S, Li M, Luo R, Wang X, Takashima A. GM-CSF-surface-modified B16.F10 melanoma cell vaccine. Vaccine 2006;24:5265–8.
- Loskog A, Ninalga C, Hedlund T, Alimohammadi M, Malmstrom PU, Totterman TH. Optimization of the MB49 mouse bladder cancer model for adenoviral gene therapy. Lab Anim 2005;39:384–93.
- Hu Z, Tan W, Zhang L, Liang Z, Xu C, Su H, . A novel immunotherapy for superficial bladder cancer by intravesical immobilization of GM-CSF. J Cell Mol Med 2010; 14:1836–44.
