Abstract
Purpose. We retrospectively compared the maximum standard uptake value (SUVmax) of FDG PET in four different sites to evaluate whether a common diagnostic SUVmax threshold may exist in these tumor locations. We further postulate that the SUVmax thresholds are higher in thoracic lesions than in extrathoracic lesions. Material and methods. N = 143 patients in four subgroups underwent a FDG PET/CT: a) 42 patients for solitary pulmonary nodules (SPNs) characterization with b) respective mediastinal lymph nodes (LNs), c) 65 patients for LN staging of head and neck cancer, and d) 36 cancer patients diagnosed with adrenal lesions. Receiver operating characteristics of SUVmax values were evaluated. Results. The SUVmax were statistically significantly greater in malignant than in benign lesions. For SPNs and mediastinal LNs, a SUVmax > 3.6 each resulted in a sensitivity of 81% and 87%, and a specificity of 94% and 89%. For cervical LNs and adrenal glands, a SUVmax > 2.2 each showed a sensitivity of 98% and 100%, and a specificity of 83% and 93%. Conclusion. A common SUVmax threshold did not exist in the four studied subgroups. The variable FDG uptake in SPNs and mediastinal LNs are associated with the high prevalence of inflammation/infection within the chest. Similar SUVmax thresholds however may exist for extrathoracic regions where the prevalence of inflammation/infection is low.
Computed tomography (CT) and fluorine-18 fluorodeoxyglucose positron emission tomography (FDG PET) are imaging modalities that play an important role in the diagnosis and staging of various cancers [Citation1]. Standard uptake value (SUV) as a semi-quantitative measure points to the degree of metabolic activity in the abnormal tissues. Reports have shown a wide range of maximum SUV (SUVmax) cut-off values for various tumor entities. For example, SUV thresholds ranging from 2.0 to 3.8 have been proposed to separate malignant from benign lung nodules [Citation2,Citation3]. SUVmax thresholds of 1.9 to 3.0 have been reported for cervical LN staging [Citation4]. So far, published data about FDG PET are confined to the use of SUVmax for single tumor entities and site locations. Because of the variations in the study populations, scanner systems and scanning protocols, a clear conclusion about a common diagnostic SUV threshold cannot be drawn.
We carried out a retrospective study using data from a single institution and sought to determine and correlate the SUVmax thresholds of FDG PET/CT for characterization of SPNs, with respective mediastinal LNs, and cervical LN staging in head and neck cancer and characterization of adrenal lesions in cancer patients. We further postulate that the SUVmax thresholds are higher in thoracic lesions than in extrathoracic lesions.
Material and methods
Patients
We included four clinical scenarios in this study for which FDG PET has proven efficacy in diagnosing cancer. Characterization of SPN and staging of head and neck cancer were chosen because these were typical indications previously approved by Medicare and Medicaid Services (CMS). We also included the characterization of mediastinal lymph nodes in patients with SPN, which was comparable with mediastinal staging of lung cancer. Adrenal lesions were included because FDG PET has shown to have high diagnostic accuracy to distinguish between benign and malignant lesions [Citation5,Citation6].
A total of 143 patients undergoing F-18 FDG PET/CT scans between September 2004 and October 2007 were selected because there was either histopathologic confirmation of the diagnosis or imaging follow-up. The four patient subgroups were as follows: a) 42 patients for characterization of SPN measuring 1 to 3 cm in size and b) of their mediastinal LNs – 30 men, 12 women, mean age of 64 years, 48–82 years; c) 65 patients for staging of head and neck cancer – 50 men, 15 women, mean age of 58 years, 40–78 years; and d) 36 cancer patients with adrenal gland lesions by PET/CT – 16 men, 20 women, mean age of 66 years, 41–81 years.
Standard references
Histopathology was available in all 42 SPNs. In biopsy proven lung cancer, the etiology of hilar and mediastinal LNs, either benign or malignant, were also verified with biopsy. If the lung nodule was benign by histopathology, the mediastinal LNs in the same patient were considered benign and biopsy was not performed. In all patients diagnosed with head and neck cancer, histopathologic confirmation was available for the cervical LN regions included in this study. The nature of adrenal lesions was verified by histopathology or imaging follow-up including PET/CT, diagnostic CT and MRI. The study was approved by the Human Research Committee of our institution.
PET/CT scanning
The patients fasted at least four hours before the PET/CT examination and received an intravenous injection of about 5.18 MBq/Kg (0.14 mCi/Kg) of 18F-FDG, with a maximum of 444 MBq (12 mCi). Blood glucose concentration was < 200 mg/dl immediately before the tracer injection in all studied subjects. Patients sat in a quiet injection room and were instructed not to talk during the following 60 min of the FDG uptake phase. All scans were acquired during normal breathing.
From 9/2004 to 8/2006, 115 of the 143 patients underwent their exam on a Gemini scanner (Philips Medical Systems) and the remaining 27 patients underwent their exam on a Gemini TF scanner (Philips Medical Systems), from 9/2006 until 10/2007. The Gemini scanner and Gemini TF have an axial co-scan range of 185 cm and 193 cm enabling a head-to-toe imaging in one sweep; the CT consisted of 16-slice (Gemini) and 64-slice (Gemini TF) multi-detector helical CT and was performed before the PET scan. The low-dose CT data were used for generation of the CT transmission map, image fusion and anatomical localization of FDG foci and measurement of lesion size. No oral or intravenous contrast was used. PET images were acquired at three minutes per bed position for Gemini and two to three minutes per bed position for Gemini TF depending on the patient's weight.
Image analysis
The PET/CT images were evaluated on the Extended Brilliance workstation for the Gemini TF (Philips Medical Systems). The SUVmax of the SPNs as seen on CT, the SUVmax of the mediastinal and cervical LNs with the highest FDG uptake, and the SUVmax of both adrenal glands, at least one of which was nodular on CT, were measured. SUVmax was calculated as the ratio of the maximum regional radioactivity concentration divided by the injected amount of radioactivity normalized to body weight. At each image slice, a circular region-of-interest was manually positioned around the lesions, and the SUVmax within that region-of-interest was computed. In lesions with no obvious FDG uptake, the region-of-interest was placed around the lesions as anatomically outlined by CT.
Statistical analysis
Patient data were assessed using measures of central tendency (median, minimum-maximum) and frequencies (%) for categorical variables. Mann-Whitney tests were used to compare the size and SUVmax between benign and malignant lesions. Cut-off values and areas under the curves (AUC) for SUVmax were calculated using receiver operating characteristics (ROC). A two-sided p-value of < 0.05 was considered statistically significant. The statistical software MedCalc®, Version 9.3.0.0 was used.
Results
Etiology of lesions
summarizes the distribution of benign and malignant lesions in the four subgroups. SPNs and cervical LNs showed high incidence of malignancy (≥ 60%) compared with that of mediastinal LNs and adrenal gland lesions (≤ 35%).
Table I. Summary of the distribution of benign and malignant lesions in the four subgroups. Data are numbers (%) of patients and on a patient-by-patient basis unless otherwise indicated.
The cell types of the 42 SPNs were as follows: 13 adenocarcinoma (31%), four squamous cell carcinoma (10%), two neuroendocrine carcinomas (5%), one large cell carcinoma (2%), one small cell carcinoma (2%), five carcinomas, type not specified (12%), nine fibrosis/inflammation (21%), four infection (10%), and three benign tumors (7%). With regard to their respective mediastinal staging, 15 of 42 patients (36%) and 27 of 42 patients (64%) were found to have malignant and benign lymph nodes, respectively. Twenty-two of the 42 patients with SPN (52%) underwent mediastinal biopsy, showing metastasis in 14 patients (64%) and benign findings in eight patients (36%) – inflammatory and infectious etiologies was noticed in six patients (75%) and normal lymphoid tissue was seen in two patients (25%). The median follow-up for the SPNs and respective mediastinal LNs was five weeks, range 1–105.
For head and neck cancer, 63 of 65 (97%) patients presented with squamous cell carcinoma. Detailed biopsy results were available in 20 of 25 patients (80%) that had benign cervical LNs; reactive inflammatory LNs were found in three patients (15%) and benign lymphoid tissue was seen in 17 patients (85%). Forty of 65 patients (62%) were found to have metastatic cervical LNs, of which 35 patients (88%) had biopsy proven disease. Median follow-up for cervical LNs was three weeks, range 1–122.
On a lesion-by-lesion basis, 58 of 72 (81%) and 14 of 72 (19%) adrenal glands were benign and malignant, retrospectively (). These 14 malignant lesions derived from 11 patients – eight patients had unilateral lesions and three patients had bilateral lesions. On a patient-by-patient basis, 25 of 36 patients (69%) were found to have benign adrenal lesions, which were verified with histopathology in five patients (20%), all showing no evidence of inflammation or infection. Malignant adrenal lesions were found in 11 of 36 patients (31%) deriving from six lung primaries, two malignant melanomas, one head and neck cancer, one breast cancer and one germinal carcinoma; histopathologic confirmation was available in two patients (18%), and in the remaining nine patients (82%), the malignant etiology was concluded based on clinical and radiographic follow-up. Median follow-up for the adrenal glands was 53 weeks, range 2–166.
Lesion sizes and SUVmax thresholds
summarizes the findings of lesion size and SUVmax of the four subgroups. The size and SUVmax were statistically significantly greater (p < 0.05) in malignant than in benign lesions for three of four subgroups; only the size of the SPNs that was not statistically significant (p = 0.62) between malignant and benign lesions. Box-and-whisker plots of SUVmax for the four subgroups are summarized in . SUVmax between benign and malignant lesions of SPNs and mediastinal LNs overlapped more than in those of cervical LNs and adrenal glands.
Figure 1. Box-and-whisker plots of SUVmax of SPN, a: mediastinal LNs, b: cervival LNs, c: and adrenal glands, d: Closed dots represent mild outliers. The frequency of extreme outliers shown as open dots is seen only in SPNs and mediastinal LNs and is absent in cervical LNs and adrenal glands. Moreover, SUVmax between benign and malignant lesions of SNPs and mediastinal LNs overlap more than in those of cervical LNs and adrenal glands. As a result, SUVmax thresholds are positively skewed for SPNs and mediastinal LNs. Data are on a patient-by-patient basis except the adrenal glands which are on a lesion-by-lesion basis.
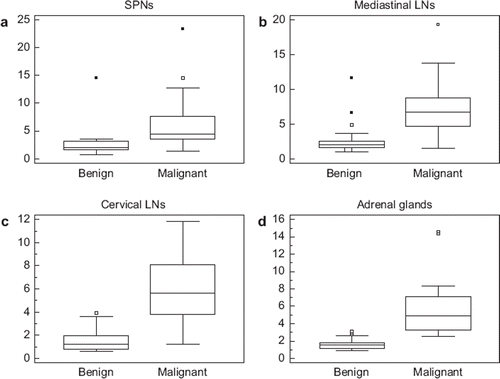
Table II. Summary of lesion size and SUVmax characteristics in the four subgroups. Data for lesion size and SUVmax are median (minimum-maximum) and on a patient-by-patient basis unless otherwise indicated.
The SUVmax cut-offs and AUCs and corresponding sensitivities and specificities and well as positive predictive values and negative predictive values for all four subgroups are presented in . FDG PET/CT showed lower diagnostic accuracies for SPNs and mediastinal LNs, with AUC of 0.853 (95% confidence interval (CI), 0.71–0.943) and 0.889 (95% CI, 0.753–0.964), and greater diagnostic accuracies for cervical LNs and adrenal glands, with AUC of 0.959 (95% CI, 0.877–0.992) and 0.990 (95% CI, 0.929–0.997). Negative predictive values of 75% for SPNs and 92% for mediastinal LNs were lower compared with 97% for cervical LNs and 100% for adrenal glands. A false-positive PET/CT case of lung cancer and mediastinal metastasis is illustrated in .
Figure 2. A 71-year-old man was admitted with delirium and fever. An MRI of the brain with and without contrast showed subacute ischemic of the left posterior frontal and parietal lobes. Blood cultures, lumbar puncture and bronchoalveolar lavage were negative for infectious pathogens. Diagnostic CT of the chest showed a right lower lung lobe nodule and an enlarged right tracheobronchial lymph node for which the FDG PET/CT was ordered for further evaluation. Axial PET (a), CT (b) and fused PET/CT (c) images demonstrated a spiculated 2.5 × 1.2 cm right lower lung lobe nodule with prominent FDG uptake (SUVmax 3.8), short arrow. This lesion (short arrow) was seen in the maximum-intensity-projection (MIP) image (d), which also showed a hypermetabolic focus in the right tracheobronchial region (long arrow). Axial PET (e), CT (f) and fused PET/CT (g) demonstrated an intensely hypermetabolic (SUVmax 4.2) right tracheobronchial lymph node measuring 1.7 × 1.5 cm in size (long arrow). The PET/CT findings were suspicious for lung primary with mediastinal metastasis. Subsequent core biopsy of the right lung nodule revealed granulomatous disease with abundant yeasts. Following antifungal treatment, the patient was afebrile on 3-month follow-up, and the right lung nodule and mediastinal lymph node resolved on chest CT.
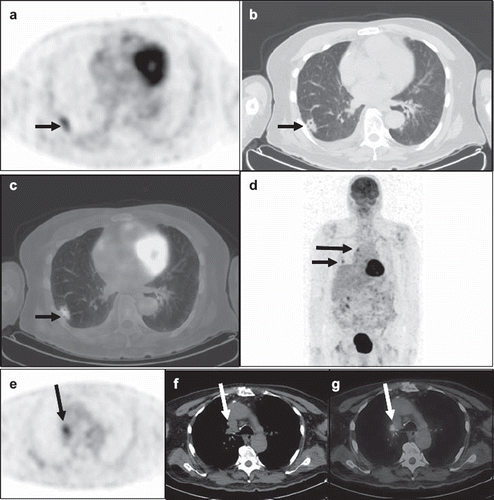
Table III. Summary of SUVmax thresholds and corresponding sensitivities, specificities, positive predictive values (PPV), negative predictive values (NPV) as well as AUC for the four subgroups. Data are on a patient-by-patient basis unless otherwise indicated.
Discussion
Solitary pulmonary nodules
Kim et al. [Citation2] recently reported that PET/CT had a sensitivity and specificity of 97% and 85% for characterization of SPN measuring up to 3 cm in size. The size criteria in the current study are similar, with the sizes ranging from 1–3 cm in size. Also, the cell types of lung cancer were similar in our study and the one by Kim et al – adenocarcinoma 31% vs. 44%, squamous cell carcinoma 10% vs. 11%, neuroendocrine carcinomas 5% vs. 19%, and not specified carcinoma 12% vs. 15%. Although the malignancy rate in the current study (62%) is similar to that published by Kim et al. (69%), the sensitivity in the current study is lower, 81% compared with 97%. The difference in the sensitivity is probably in part because of the different metabolic features for both benign and malignant lesions in both studies. For malignant lesions, the median SUVmax was 4.5 (range 1.4–23.4) in the current study and the average SUV was 3.0 ± 3.0 (range 0.5–17.2) in the article by Kim et al. Benign lesions in the current study showed a median SUV of 2.0 (0.6–14.5), however, no comparable data are available in the study by Kim et al.
Our SUVmax results also agree with those recently published by Joeng et al. [Citation7] in which the SUVmax greatly overlapped between benign and malignant SPNs; median SUV in that study was 2.3 (range 0.5–10.6) for benign SPNs and was 6.6 (range 2.0–18.7) for malignant SPNs, p < 0.01. Moreover, the SUVmax threshold of 3.6 in our study is a result of positively skewed data that results from inflammatory and infectious lesions (). The FDG uptake was considerably high in one patient with caseating granulomatous inflammation with a SUVmax of 14.5.
Mediastinal lymph nodes
FDG PET scanning is helpful not only to characterize SPN but also to evaluate mediastinal LNs [Citation8,Citation9]. ROC analyses in several studies have shown that SUVmax thresholds in FDG PET may range from 3.5 to 5.3 in mediastinal LNs [Citation8–10], with sensitivities of 89–91% and specificities of 84–88% which are comparable with SUVmax threshold of 3.6, sensitivity of 87% and specificity of 89% found in the current study.
Cervical lymph nodes
The SUVmax threshold of 2.2 for cervical LNs in the current study agrees with that published by Murakami R et al. [Citation4]. These authors showed that SUVmax thresholds might range from 1.9 to 3.0, depending on the size of the cervical LNs, with the smaller lesions having lower SUVmax thresholds compared with the larger ones. They found an overall sensitivity of 79% and specificity of 99% for the various SUVmax thresholds. In comparison, our findings showed a greater sensitivity of 98% but a lower specificity of 83%, which might be attributed to a greater percentage of malignant LNs in the current study (62% compared with 17%).
Adrenal glands
The 81% prevalence of benign adrenal glands on a lesion-by-lesion basis and 69% on a patient-by-patient basis in the current cohort appears comparable with that in previous studies ranging from 61–78% [Citation5,Citation6]. Reported SUVmax range from 2.7 to 3.1 was found to be highly accurate to characterize adrenal lesions, with sensitivities ranging from 98–100% and specificity ranging from 78–92% [Citation5,Citation6]. We have found similar sensitivity and specificity of 100% and 93% but our SUVmax threshold of 2.2 was lower than previously reported. In contrast to published data, we assess not only the adrenal gland that is enlarged but also the contralateral adrenal gland normal in size by CT. We include normal sized adrenal glands because it has been shown that even a normal sized adrenal gland may also harbor malignancy [Citation5,Citation6]. In the current study, the median size for benign, normal-sized adrenal gland was 0.7 cm, range 0.3–3.9 cm, and median size for malignant adrenal lesions was 2.7 cm, range 1.2–4.2 cm. These sizes are smaller compared with data previously published in which the sizes ranged from 0.5–11.0 cm in diameter [Citation5,Citation6].
Similar SUVmax ranges of SPNs and mediastinal LNs vs. cervical LNs and adrenal glands
Current reports have shown a wide range of SUVmax thresholds for various single tumor entities and site locations. Moreover, the studies were carried out in different institutions, which is associated with variations in study populations, scanner systems and scanning protocols. Thus, a clear conclusion about a common diagnostic SUV threshold cannot be drawn. Because the data of our study derive from a single institution, associated confounding factors of study populations, scanner systems and scanning protocol are kept to a minimum. The correlation SUVmax with tumor site locations appears appropriate for generalization and definition of common SUV thresholds in FDG PET scanning.
The SUVmax ranges of benign and malignant lesions in the current four subgroups are overall comparable with previously published data [Citation4–9]. However, the SUVmax greatly overlap between benign and malignant SPNs, as well as benign and malignant mediastinal LNs. The associated high SUVmax thresholds are attributed to the high prevalence of inflammatory and infectious processes within the chest, as verified by biopsy in 31% of SPNs and in 75% of mediastinal LNs. In contrast, the SUVmax overlap less between benign and malignant cervical LNs, as well as benign and malignant adrenal glands. This finding is associated with the low prevalence of inflammatory and infectious processes, as found in only 15% for cervical LNs and none for adrenal lesions.
Practical considerations
It would be of great practical value if there were a common diagnostic SUVmax threshold for FDG PET above which malignancy could be diagnosed with confidence. Our results show however that a common diagnostic SUVmax does not exist for SPNs and respective mediastinal LNs, as well as LN staging of head and neck cancer and adrenal nodules. Rather, SUVmax thresholds may vary depending on the metabolic activity of the lesions but also on the prevalence of inflammation and infection in a specific region of the body.
Because of the comparable prevalence of inflammation and infection within the chest, the SUVmax thresholds for SPNs and mediastinal LNs may be similar or even identical as found in the current study with SUVmax > 3.6 each. The FDG uptake may vary in inflammatory and infectious lesions, which makes them difficult to be distinguished from malignant lesions [Citation9,Citation11]. Thus, the use of SUVmax threshold for chest lesions may be less reproducible and reliable.
The diagnostic accuracy of SUVmax thresholds for cervical LNs and adrenal lesions in the current study was excellent with AUC > 0.95 and identical SUVmax threshold of 2.2. These regions of the body harbor less inflammation and infection, as shown in 15% in head and neck cancer patients and in none of the patients with benign adrenal nodules.
In practice, the use of SUV in FDG PET to diagnose cancer is an issue of ongoing controversy. Interpretation of FDG PET is usually based on visual evaluation and not on SUV measurements [Citation2,Citation9] because data have shown that the use of SUV failed to be more accurate than the visual evaluation in predicting the presence of malignancy. One explanation is that ROC analyses often reveal SUVmax cut-offs that are greater than 3.0. This relatively high SUVmax threshold may be associated with decreased negative predictive values [Citation9], as was seen in our patients with SPNs and mediastinal LNs. The use of SUVmax thresholds drawn from ROC analyses therefore might lead to clinically suboptimal FDG PET diagnoses and might serve as complementary tool beside visual evaluation [Citation9]. Nevertheless, the knowledge of different SUVmax thresholds in different parts of the body as shown in the current study may help increase confidence and accuracy in FDG PET/CT interpretation. Particularly, the information that SUVmax thresholds are consistently greater for thoracic lesions than those extrathoracic is useful and may help avoid misdiagnosis.
Besides, the likelihood that increased FDG uptake indicates malignancy within a lesion is influenced by the patient characteristics (e.g. age, history of smoking, history of cancer, and other factors), the presence of comorbidity (e.g. granulomatous disease), and the appearance of the lesion on CT, especially when reading a PET/CT scan. PET/CT interpretation should therefore include all relevant clinical and radiological information specific to the patient in question, together with an appreciation of the clinical implications of the FDG avidity of the lesion.
Limitations
We acknowledge the limits of our retrospective study that contains a relatively small number of patients in each patient subgroup. The differences in the lesion sizes and prevalence of benign vs. malignant lesions between the four subgroups may have affected the results of the SUVmax thresholds. This possibility however is less likely because the prevalence of benign lesions is not much different in body regions with higher SUVmax thresholds, as was the case for 38% of SPNs and 64% of mediastinal LNs, compared with body regions with lower SUVmax thresholds, as seen in 38% of cervical LNs and 81% of adrenal lesions. Also, the sizes of the benign lesions do not differ much between mediastinal LNs (median 1.0 cm, range 0.4–2.0), cervical LNs (median 0.9 cm, range 0.3–1.5) and adrenal gland lesions (median 0.7 cm, range 0.3–3.9). SPNs are an exception because of predefined inclusion size 1–3 cm in the current study, which represents a selection bias.
Another limitation may be that the SUVmax of only one mediastinal LN and one cervical LN with the highest FDG uptake was measured although the patients might have had several enlarged LNs. This may be important because metastatic disease may not be present in a measured LN with high SUV but may be present in another LN nearby that has a lower SUV.
The assessment of SUVmax thresholds is limited to two locations each for thoracic and extrathoracic lesions. The evaluation of SPNs and mediastinal LNs for thoracic region is necessary. For extrathoracic region, we select cervical LN and adrenal lesions because of their clearly defined anatomical locations and the high diagnostic accuracy of FDG PET. We may have chosen other indications for extrathoracic locations such as malignant melanoma or lymphoma, for which however, the primary sites and metastatic lymph node stations may vary greatly in locations. Because of the heterogeneous distribution of lesions within the body, malignant melanoma or lymphoma appears suboptimal for our study design as compared to cervical LNs for head and neck cancer staging and adrenal characterization of patients with history of malignancy. Nevertheless, a broader spectrum of site locations such as inguinal lymph nodes and additional clinical indications such as response to therapy evaluation may be more appropriate.
The different scanner systems used in this study may have affected the SUVmax because of their scintillator types, crystal sizes and image reconstruction method [Citation12]. The spatial resolution is better with the Gemini TF scanner than with the Gemini scanner. However, the injected FDG dose, time of uptake phase and image reconstruction method were comparable between Gemini and Gemini TF [Citation13,Citation14]. The differences in SUVmax characteristics between these two latest scanner systems are smaller than would have been expected from the use of an older and a newer scanner system. Nevertheless, the measured SUVmax values are mainly valid for the scanner equipment and scanning procedure being used and may vary for different PET scanners and procedures.
Conclusions
A common SUVmax threshold in FDG PET did not exist in the four studied subgroups. The variable SUVmax values seen in SPNs and mediastinal LNs are attributed to the variable metabolic features of inflammation and infection within the chest, which may result in less desirable diagnostic accuracy. The high diagnostic accuracy with identical SUVmax values found in cervical LNs and adrenal lesions suggests that similar SUVmax thresholds may exist, particularly for regions outside the chest where there is a lower prevalence of inflammation and infection.
Acknowledgements
No research support was granted. The authors report no conflict of interest.
References
- Czernin J, Allen-Auerback M, Schelbert HR. Improvements in cancer staging with PET/CT: Literature-based evidence as of September 2006. J Nuc Med 2004;48(Suppl 1): 78S–88S.
- Kim SK, Allen-Auerbach M, Goldin J, Fueger BJ, Dahlbom M, Brown M, . Accuracy of PET/CT in characterization of solitary pulmonary lesions. J Nucl Med 2007;48: 214–20.
- Hübner KF, Buonocore E, Gould HR, Thie J, Smith GT, Stephens S, . Differentiating benign from malignant lung lesions using “quantitative” parameters of FDG PET images. Clin Nucl Med 1996;21:941–9.
- Murakami R, Uozumi H, Hirai T, Nishimura R, Shiraishi S, Ota K, . Impact of FDG-PET/CT imaging on nodal staging for head-and-neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys 2007;68:377–82.
- Blake MA, Slattery JMA, Kalra MK, Halpern EF, Fischman AJ, Mueller PR, . Adrenal lesions: Characterization with fused PET/CT image in patients with proved or suspected malignancy – Initial experience. Radiology 2006;238: 970–7.
- Metser U, Miller E, Lerman H, Lievshitz G, Avital S, Even-Sapir E. 18F-FDG PET/CT in the evaluation of adrenal masses. J Nucl Med 2006;47:32–7.
- Jeong SY, Lee KS, Shin KM, Bae YA, Kim BT, Choe BK, . Efficacy of PET/CT in the characterization of solid and partly solid solitary pulmonary nodules. Lung Cancer 2008;61:186–94.
- Bryant AS, Cerfolio RJ, Klemm KM, Ojha B. Maximum standard uptake value of mediastinal lymph nodes on integrated FDG-PET-CT predicts pathology in patients with non-small cell lung cancer. Ann Thorac Surg 2006;82:417–22.
- Hellwig D, Graeter TP, Ukena D, Groeschel A, Sybrecht GS, Schaefers HJ, . 18F-FDG PET for mediastinal staging of lung cancer: Which SUV threshold makes sense? J Nucl Med 2007;48:1761–6.
- Yi CA, Lee KS, Kim BT, Shim SS, Chung MJ, Sung YM, . Efficacy of helical dynamic CT versus integrated PET/CT for detection of mediastinal nodal metastasis in non-small cell lung cancer. AJR 2007;188:318–25.
- Bury T, Dowlati A, Paulus P, Corhay JL, Benoit T, Kayembe JM, . Evaluation of the solitary pulmonary nodule by positron emission tomography imaging. Eur Respir J 1996;9: 410–4.
- Westerterp M, Pruim J, Oyen W, Hoekstra O, Paans A, Visser E, . Quantification of FDG PET studies using standardised uptake values in multi-centre trials: Effects of image reconstruction, resolution and ROI definition parameters. Eur J Nucl Med Mol Imaging 2007;34:392–404.
- Gregory R, Partridge M, Flower MA. Performance evaluation of the Philips “Gemini” PET/CT system. IEEE Trans Nucl Sci 2006;53:93–101.
- Surti S, Kuhn A, Werner ME, Perkins AE, Kolthammer J, Karp JS. Performance of Philips Gemini TF PET/CT scanner with special consideration for its time-of-flight imaging capabilities. J Nucl Med 2007;48:471–80.
