Abstract
Purpose. Survivors after malignant lymphoma are at high risk of late effects. In order to take full responsibility for their own health they need knowledge about their diagnosis, treatment and risk of late effects. We assessed such knowledge in adult survivors of childhood malignant lymphoma. Material and methods. In 2007–2009 128 five-year survivors after childhood malignant lymphoma participated in a national cross-sectional questionnaire-based survey combined with clinical examination. [Males: 69, females: 59, treatment period 1970–2000, median age (range) at diagnosis: 14 years (0–18), at survey: 32 years (19–55), Hodgkin lymphoma (HL): 84, Non-Hodgkin lymphoma (NHL): 44]. Prior to the clinical examination a semi-structured interview on the survivors’ knowledge was conducted by a study nurse. The individual survivors’ responses were compared with his/her medical record. Results. One hundred and twenty one reported their diagnosis correctly, seven reported that they had cancer, but could not specify malignant lymphoma. Thirty-three could not differentiate between HL and NHL. One hundred and twenty three reported their treatment modalities correctly (radiotherapy vs. chemotherapy vs. combined). Eighty-five (66%) were not aware of any risks for late effects. The remaining 43 listed at least one of the following late effects; infertility, heart-problems, impaired dental status, hypothyroidism, breast cancer, reduced muscle growth, fatigue and reduced memory or concentration. Thirty-seven survivors who provided additional comments reported that they had received some information about risk of late effects from their therapists. Age at diagnosis or educational level were not associated with knowledge about possible late effects while treatment period was. Conclusions. Norwegian long-term survivors of childhood malignant lymphomas are showing improved level of knowledge of their diagnosis and treatment modalities during the last decade. Still, independent of age at diagnosis and level of education, they are insufficiently aware of their risk of late effects.
Background
Advances in the treatment of pediatric malignancies during the last decades have increased the numbers of childhood cancer survivors substantially [Citation1]. However, these survivors are at increased and persistent risk to develop late effects [Citation1,Citation2]. Late effects might increase mortality and/or impair quality of life [Citation3,Citation4]. Some late effects, such as gonadal dysfunction, may be recognized few years after treatment completion, whereas others, such as cardiovascular late effects, commonly become manifest after 10–20 years or more [Citation4].
In the Nordic countries, pediatric cancer survivors are usually followed at the pediatric departments until the age of 18 years. Subsequent follow-up is in general less organized, and contact with the health care system primarily becomes the responsibility of the adult cancer survivors. A report from the UK indicates that most survivors of childhood cancers are not followed on a regular basis for the purpose of preventing, detecting and treating late effects [Citation5]. This is also the case in Norway at present.
In order to take adequate responsibility for their health, childhood cancer survivors have to be aware of their disease, treatment and risk for late effects. Such knowledge should encourage them to participate in recommended screening programs and adapt to a healthy lifestyle including optimal nutrition, cessation of smoking, physical training and weight control. Further, when contact with the health care system is established in adult life, knowledge about previous disease and treatment is valuable in relation to subsequent health risk assessments and diagnostic and therapeutic decisions. For example, knowledge about thoracic radiotherapy during childhood or adolescence is necessary for the family doctor to prescribe regular mammography in females. Further, in many instances, information about past disease and treatment can only be provided by the survivors themselves when they are in contact with the health care system [Citation6].
Malignant lymphoma, most often non-Hodgkin lymphoma (NHL) is the third most common malignancy in children and adolescents with 5–10 new cases/year per million children or adolescents [Citation7]. Treatment includes radiotherapy and/or chemotherapy, the latter containing anthracyclines from the mid-80s. Relative 10-year survival rates for childhood NHL are approximately 80% and above 90% for Hodgkin's lymphoma (HL) [Citation8]. On the other hand, during the last two decades increasing evidence has emerged on the risk of late effects including premature death 20–30 years after treatment [Citation9,Citation10]. In spite of this, no part of the health care system has a defined obligation to provide long-term survivors with relevant information about late effects.
In 2002, Kadan Lottick et al. reported that childhood cancer survivors in USA had insufficient knowledge about their risk of late effects [Citation10]. These findings cannot be directly transferred to European settings due to societal differences and differences in health care systems. Further, one may also question whether awareness about late effects among childhood cancer survivors has improved during the last decade. Programs for informing about, detecting and treating late effects might also differ between hospitals. The Nordic Cancer Registries provide a unique possibility to identify unselected national samples of cancer survivors and, even many years after treatment, reach them and inform them about their risk of late effects, preventive actions and how to diagnose and treat the late effects.
In the present study we studied knowledge about diagnosis, treatment and risk of late effects among long-term survivors of pediatric malignant lymphomas treated in Norway during the last three decades of the 20th century. Based upon clinical experience and previous international studies [Citation5,Citation10,Citation11] we hypothesized that only a minority would have sufficient knowledge about their risk of late effects. Secondarily we aimed at identifying factors which characterize survivors with such knowledge.
Material and methods
In the period 1970–1999, treatment of childhood cancers in Norway was principally administered by the pediatric department at the five national university hospitals. However, and in particular during the 70s, children and adolescents with malignancies requiring extended radiotherapy were also hospitalized at the Norwegian Radium Hospital (NRH), a Comprehensive Cancer Center which mainly treats adult-onset cancers.
In 2007 Norwegian adult long-term cancer survivors treated during childhood and adolescence for histologically verified malignant lymphoma were invited to the present study by the Cancer Registry of Norway. Their identity was cross-checked by pediatricians working at the responsible pediatric units. The pediatricians then invited the eligible survivors to participate in a cross-sectional questionnaire-based survey combined with a clinical examination performed at the Norwegian Radium Hospital (NRH). One reminding letter was sent to those who did not respond to the initial. Eligible survivors of HL had been treated at one of the five Norwegian university hospitals during 1970–2000 or they had been treated for NHL at the NRH during the same period. Additional eligibility criteria for all subjects were age at first cancer diagnosis ≤18 years and age at survey >18 years.
The mailed questionnaire, to be filled in at home prior to the out-patient clinical examination, consisted of validated instruments and single items assessing health-related quality of life, specific health problems, educational level and work ability. The clinical examination was combined with echocardiography and testing of bone density, vision and cognitive function in addition to blood tests. The results of these examinations will be published as separate papers.
The present study is based on a semi-structured interview conducted by the responsible study nurse and performed immediately prior to the clinical examination. The content of the interview was based on a Norwegian translation of the questions and response alternatives previously used by Kadan-Lottich et al. [Citation10] in a study of childhood cancer survivors on their knowledge about diagnosis (A), treatment (B), risk of late effects (C) and follow-up and received information (D) (). The individual survivor's responses were compared to his/her medical record.
Table I. Key questions of the semi-structured interview.
Responses to question C about the survivor's knowledge of his/her risks for late effects were defined as the primary endpoint of this study. The response alternatives were “Yes”, “No” or “Uncertain” (the latter finally treated as “No” in the analyses). As a follow-up to question C the patients were encouraged to further explain their responses. Based upon these comments, the sources for information on late effects were categorized into: the treating pediatric or oncological department, family doctors, Internet/media, other childhood cancer survivors or own experience of health problems.
Data analysis
Standard descriptive statistics were applied for all variables (median, range and frequencies). Differences in characteristics between groups were analyzed with Chi-square tests. The significance level was set to p-values ≤ 0.05. Analyses were performed by the SPSS 16.0 (SPSS Inc., Chicago, Il, USA).
Ethics
The Regional Committee for Medical and Research Ethics and the Norwegian Data Inspectorate approved the study. All participants gave written informed consent. All participants received a summary of the findings from their examination and in case of untreated late effects, referrals to adequate health care were done.
Results
A total of 223 survivors were invited to participate, and 130 survivors (58%) accepted. Ninety-three did not want to attend the out-patient consultation or did not reply at all. Of the 130 survivors who attended the out-patient consultation, two survivors were subsequently excluded because review of the medical records revealed that their diagnoses of malignant lymphoma were incorrect. Thus, 128 eligible survivors after malignant lymphoma were interviewed by the study nurse. The demographic and medical data, as obtained from the Cancer Registry, were comparable between patients who participated in the study and those who did not (data not shown).
Eighty-four (66%) of the 128 participating survivors had been treated for HL and 44 (34%) for NHL (). Sixty-nine survivors (54%) were males. Median age at diagnosis was 14 years (range 0–18), and median age at the time of the study was 32 years (range 19–55). Median time since diagnosis (observation time) was 20 years (range 7–37). One third of the patients had received chemotherapy only, 58% had received both chemotherapy and radiotherapy and 9% radiotherapy only. Fifty percent of the survivors reported more than 12 years of education.
Table II. Demographics of participants.
Knowledge about diagnosis
Totally, 121 survivors (95%) confirmed that they had had “malignant lymphoma” (). The remaining seven survivors reported “cancer”, but not malignant lymphoma. Of these, five survivors reported respectively brain tumor, cancer of the bone marrow, bowel or salivary gland and epiglottis which corresponded to the main localization of their lymphoma, where as two survivors knew they had had a “malignant disease” without reporting additional details.
Table III. Responses to key questions.
Among the 121 survivors who were aware of their prior diagnosis of malignant lymphoma, 71 of 82 HL survivors (87%) and 17 of 39 NHL survivors (44%) could correctly state their type of malignant lymphoma (HL vs. NHL; p < 0.001). The comparable figure among NHL-survivors was 44% (17 of 39 survivors). Females were significantly more often aware of their type of malignant lymphoma than males (82% of the females vs. 64% of the males, p < 0.05). Median age at diagnosis was higher among those who knew their type of lymphoma compared to those who did not (HL vs. NHL, 15 (range 2–18) vs. 12 years (range 0–18), p < 0.05). Educational level, age at time of study and observation time were not significantly related to knowledge of the type of malignant lymphoma.
Knowledge about treatment
A total of 123 survivors (96%) correctly reported their treatment modality (radiotherapy, chemotherapy or combined) without any difference between survivors of HL and NHL (). Localization of radiation sites were reported with high accuracy: 80 of 86 survivors (93%) precisely described the localization of the irradiated areas. The five survivors who had received total body irradiation correctly reported this treatment.
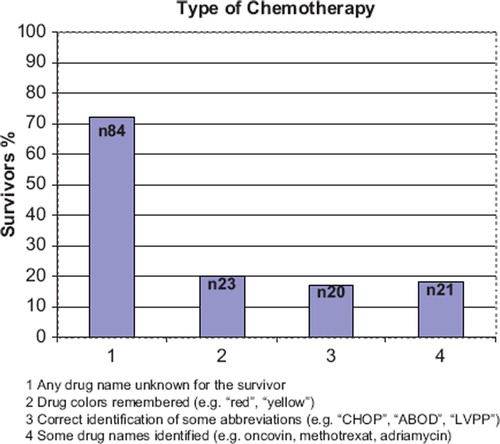
Eighty-four of 116 (72%) survivors who had received chemotherapy could not name any of the cytostatic drug they had received (). Twenty-three (20%) remembered the colors (red or yellow) and 20 (17%) could name some acronyms for the chemotherapy regimen (e.g. “CHOP”, “ABOD”, “LVPP”). Only 21 survivors (18%) could correctly mention at least one cytostatic drug (e.g. oncovin, methotrexat).
Knowledge about the risk of late effects
When asked if they had been made aware of any late effects after treatment, 85 (66%) answered “No” (). The remaining 43 (34%) reported that they knew about at least one late effect. The proportion of survivors knowing about the risk of late effects increased by treatment period. More patients treated after 1989 had some knowledge about the risk of late effects (p < 0.01) compared to those treated before 1990 (). A higher percentage of the survivors treated at pediatric departments were aware of late effects compared to those treated at the NRH (p < 0.09). Gender, age at diagnosis, educational level and type of malignant lymphoma (HL vs. NHL) were not significantly related to awareness of late effects.
Table IV. Factors associated with the awareness of late effects.
Based on the comments provided by 75 survivors (37 males, 38 females) in connection to question C, gonadal dysfunction was the most frequently reported late effect (27 of 75 survivors with comments; 36%) (data not shown). Less than 20% of the survivors reported that they had been informed about risks for cardiovascular, dental, pulmonary and thyroid morbidities. Only seven female patients were aware about their risk of breast cancer, representing 18% of the females who had received thoracic irradiation. These patients had participated in a previous screening study for breast cancer organized by the NRH [Citation12].
Only one man stated that he had been informed about the risk of fatigue or muscular weakness, though respectively five and six subsequently acquired such knowledge from own experience.
At the subsequent clinical examination, nine patients were diagnosed with low grade aortic stenosis unknown to them prior to the study. Five patients had increased serum thyroxin stimulating hormone (TSH) not previously recognized.
Follow-up and information
A total of 112 of the 128 participants (88%) reported no current regular follow-up for possible late effects. Frequent additional comments were that the survivor had contacted their family doctor when symptoms and problems appeared. Ninety-six percent (N = 123) of the survivors had consulted a physician during the last two years, and 65 of these consultations were related to health problems that the survivors attributed to their previous treatment for malignant lymphoma. Only 15 patients (12%) confirmed that they had received a written summary of their disease and treatment. In most cases they had themselves asked specifically for such a document.
Discussion
Norwegian long-term survivors of childhood malignant lymphoma have adequate knowledge of their diagnosis and treatment modality, in particular HL survivors. However, they are insufficiently aware of their risk of late effects. Further, particularly among patient diagnosed before 1990, our findings indicate that most asymptomatic adult survivors were not informed about their health risks and that they have no contact with the health care system for regular follow-ups concerning late effects.
While the knowledge about diagnosis and use of radiotherapy seemed sufficient among our survivors, awareness of chemotherapy and its long-term risks was poorer; 72% could not list the name of any drug used in their treatment and only 6% were familiar with the name “adriamycin”. These deficits are worrying; especially concerning anthracyclines due to this drug's cardiotoxic effects.
Two-thirds of the survivors had not been aware of any risk of late effects, though with increased knowledge in those diagnosed after 1990. This observation surprises on the background that the educational level of our survivors was significantly above that of a comparable group from the Norwegian general population [13, (p <0.01)]. Similar results have been obtained in other studies [Citation10,Citation11,Citation14]. This is in our view alarming since documentation of late effects after childhood cancer has been rapidly increasing during the last 15 years. Our observations must, however, be interpreted with caution. Adequate information might have been presented, but can have been forgotten or not perceived by the survivor and/or parents. Alternatively the parents may have received information but have not passed the information to their child. Further, scientific knowledge about late effects such as second cancer or cardio-toxicity has mostly emerged during the last two decades and was therefore not available for physicians and patients during the 70s and early 80s. Our findings of a significantly higher proportion of informed patients treated in the 90s seem to support this. On the other hand, the pediatric units have had the possibility to identify survivors after malignant lymphoma treated in the 70s and 80s and could have informed them about their health risks and established preventive tasks, such as mammography.
Information about gonadal dysfunction, a relatively “early” and often easily diagnosed disorder, seems to have been better than for all other late effects. Nevertheless, 15 of our male survivors were not aware of their risk for infertility. Information about late psychosomatic effects such as fatigue and muscle weakness was close to absent, in spite of the fact that more than 25% of all adult HL survivors experience chronic fatigue [Citation15].
The majority of Norwegian long-term survivors after childhood malignant lymphoma are not enrolled in any systematic type of long-term aftercare program as for example described by Freyer et al. [Citation16]. Official recommendations for specialized screening examinations of asymptomatic cancer survivors after malignant lymphoma only include annual mammography for women who have received thoracic irradiation [Citation13]. However, only 18% of our female survivors after mediastinal radiotherapy were aware of this screening recommendation. In agreement with Oeffinger et al. [Citation17] and based on expert opinions, the Norwegian health authorities consider the family doctor as responsible for the after care of cancer survivors. Further, these shall only be referred to the specialized health care service if and when they experience specific health problems. The majority of our survivors have, indeed, received treatment for their medical problems when these emerged. The question remains open whether some of their health problems could have been avoided or minimized by specific prevention and early diagnosis.
Our findings also raise the general question when and how to inform childhood cancer survivors about their risks of late effects. The perception of such information by the cancer survivor is a prerequisite for his/her own engagement in prevention of late effects. Adolescents and young adults in general understand their disease and how their own behavior might affect their risk of impaired health [Citation14,Citation18]. Further, many childhood survivors express a wish for thorough information regarding their medical history [Citation19–21]. However, discharge from pediatric cancer follow-up at age 18 years might not be the optimal time-point for informing about health risks which may occur 20–30 years ahead. Another question is whether information to childhood cancer survivors about their risk of late effects can be perceived as harmful.
Some limitations of our study, such as the relative low response rate, should not be overseen. This might represent a bias, but comparisons of demographic factors between responders and the non-responders did not support this. Review of the non-responders’ medical records was not permitted by the Regional Ethical Committee. Secondly, we have exclusively surveyed survivors after malignant lymphoma, but do not believe that the survivorship-related problems discussed are principally different between cancer types.
Conclusions
In conclusion, our results indicate that knowledge about the risks for late effects among Norwegian survivors of childhood malignant lymphomas is surprisingly low, though improving during the last decade. They are aware of their former disease and its treatment. In order to help the survivors taking care of their own health, we think improving information about their risk of late effects is warranted. Asymptomatic survivors at high risk of late complications should be offered regular aftercare for prevention and early detection of the most severe complications. Such aftercare examinations can be organized by the family doctor working within a network of medical specialists, not at least in the case of the availability of a detailed personalized care plan. Future research should aim to define how and when relevant information should be provided to childhood cancer survivors.
Declaration of interest: It is hereby confirmed that there are no conflict of interest for any of the authors.
References
- Dickerman JD. The late effects of childhood cancer therapy. Pediatrics 2007;119:554–68.
- Wallace WH, Green DM. Late effects of childhood cancer. 1st London: Arnold; 2004.
- Kiserud CE, Loge JH, Fosså A, Holte H, Cvancarova M, Fosså SD. Mortality is persistently increased in Hodgkin's lymphoma survivors. Eur J Cancer 2010 (in press).
- Alvarez JA, Scully RE, Miller TL, Armstrong FD, Constine LS, Friedman DL, . Long-term effects of treatments for childhood cancers. Curr Opin Pediatr 2007;19:23–31.
- Taylor A, Hawkins M, Griffiths A, Davies H, Douglas C, Jenney M, . Long-term follow-up of survivors of childhood cancer in the UK. 2004;42:161–8.
- Taylor N, Absolom K, Michel G, Urquhart T, Gerrard M, Jenkins A, . Comparison of self-reported late effects with medical records among survivors of childhood cancer. Eur J Cancer 2010 (in press).
- Márky I, Schmiegelow K. Perkkiö M, Jónsson OG, Storm-Mathiesen I, Gustafsson G, . Childhood non-Hodgkin's lymphoma in the five Nordic countries. A five-year population-based study from the Nordic Society of Pediatric Hematology and Oncology. J Pediatr Hematol Oncol 1995;17:163–6.
- von der Weid NX. Adult life after surviving lymphoma in childhood. Support Care Cancer 2008;16:339–45.
- Bluhm EC, Ronckers C, Hayashi RJ, Neglia JP, Mertens AC, Stovall M, . Cause-specific mortality and second cancer incidence after non-Hodgkin lymphoma: A report from the childhood cancer survivor study. Blood 2008;111:4014–21.
- Kadan-Lottick NS, Robison LL, Gurney JG, Neglia JP, Yasui Y, Hayashi R, . Childhood cancer survivors’ knowledge about their past diagnosis and treatment: Childhood Cancer Survivor Study. JAMA 2002;287:1832–9.
- Bashore L. Childhood and adolescent cancer survivors’ knowledge of their disease and effects of treatment. J Pediatr Oncol Nurs 2004;21:98–102.
- Fosså SD, Holte H, Holmen MM, Bjøro T, Langmark F, Hess SL, . Long-term effects after mantle field radiotherapy for Hodgkin's lymphoma. Tidsskr Nor Laegeforen 2005;125:41–4.
- Fosså SD, Hess SL, Dahl AA, Hjermstad MJ, Veenstra M. Stability of health-related quality of life in the Norwegian general population and impact of chronic morbidity in individuals with and without a cancer diagnosis. Acta Oncol 2007;46:452–61.
- Blacklay A, Eiser C, Ellis A. Development and evaluation of an information booklet for adult survivors of cancer in childhood. The United Kingdom Children's Cancer Study Group Late Effects Group. Arch Dis Child 1998;78:340–4.
- Loge JH, Abrahamsen AF, Ekeberg O, Kaasa S. Hodgkins’ disease survivors more fatigued than the general population. J Clin Oncol 1999;17:253–61.
- Freyer DR. Transition of care for young adult survivors of childhood and adolecent cancer: Rationale and approaches. J Clin Oncol 2010 (in press).
- Oeffinger KC, Mertens AC, Hudson MM, Gurney JG, Casillas J, Chen H, . Health care of young adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Ann Fam Med 2004;2:61–70.
- Eiser C, Levitt G, Leiper A, Havermans T, Donovan C. Clinic audit for long-term survivors of childhood cancer. Arch Dis Child 1996;75:405–9.
- Mertens AC, Cotter KL, Foster BM, Zebrack CJ, Hudson MM, Eshelman D, . Improving health care for adult survivors of childhood cancer: Recommendations from a Delphi panel of health policy experts. Health Policy 2004;69:169–78.
- Zebrack BJ, Eshelman DA, Hudson MM, Mertens AC, Cotter KL, Foster BM, . Health care for childhood cancer survivors: Insights and perspectives from a Delphi panel of young adult survivors of childhood cancer. Cancer 2004;100:843–50.
- Skinner R, Wallace WH, Levitt GA. Long-term follow-up of people who have survived cancer during childhood. Lancet Oncol 2006;7:489–98.