Abstract
Background. Trastuzumab is a standard treatment of HER2-positive early breast cancer in many countries, and it is usually given as a one year adjuvant treatment. However, its cost-effectiveness has not been assessed in Finland. The Finland Herceptin (FinHer) trial has compared a shorter 9-week treatment protocol against no trastuzumab with promising results. The aim of this study was to assess the potential cost-effectiveness of the 9-week treatment based on the recently published five-year follow-up results of the FinHer trial. Methods. An evaluation model of breast cancer treatment was constructed using fitted survival estimates and a long-term Markov model. The cost-effectiveness of 9-week adjuvant treatment was assessed in a Finnish setting, compared to treatment without trastuzumab. The analysis was performed from a societal perspective, and a 3% discount rate was applied for future costs and outcomes. Value of information analysis was performed to estimate the potential value of further research. Results. According to the probabilistic analysis, the incremental cost-effectiveness ratio was €12 000 per quality adjusted life year (QALY), and €9300 per life year gained (LYG), when comparing adjuvant trastuzumab therapy to standard treatment without trastuzumab. The modelled incremental outcomes for trastuzumab treatment were 0.66 QALY and 0.85 LYG for a lifetime perspective. Value of information analysis showed that additional research on treatment effects would be most valuable for reducing uncertainty in the adoption decision. Conclusions. Adjuvant 9-week trastuzumab is likely to be a cost-effective treatment in the Finnish setting. Results from an ongoing trial comparing adjuvant 9-week treatment with the 12-month treatment will play a key role in addressing the uncertainty related to the treatment effect and potential cost-effectiveness of these two treatment protocols.
Breast cancer (BC) is currently the most common cancer in Finland. There were 4318 newly diagnosed cases in 2008 [Citation1] and the number is expected to rise to 5247 by 2015 [Citation2]. According to the predictions of the Finnish Cancer Registry, in 2015 BC will account for 42% of all female cancer incidence. In 2004, BC alone caused costs of €65M, and the costs are assumed to double by 2015. It is currently responsible for 12% of all cancer-related costs in Finland. Approximately 12–30% of breast cancers over-express human epidermal growth factor receptor 2 (HER2) [Citation3–5]. These HER2-positive cases are associated with a more aggressive form of disease, and in adjuvant setting they are currently treated with trastuzumab, together with conventional BC treatment.
Trastuzumab, a humanised monoclonal antibody, has proven its clinical efficacy as an adjuvant therapy in several trials [Citation6–11]. However, there is no consensus about the optimal treatment schedule or the length of adjuvant treatment. Also the duration of treatment benefit has remained unclear. Most of the randomised controlled trials have focused on 12-month treatment [Citation6–9], but also a shorter 9-week treatment regimen has been studied in Finland Herceptin trial (FinHer) [Citation10,Citation11]. In addition, a trial comparing the 9-week and 12-month trastuzumab treatment regimens is currently ongoing [Citation12].
Trastuzumab is a relatively expensive drug. It was first introduced for the treatment of HER2-positive metastatic BC, but later the indication was extended to adjuvant treatment in early stage of the disease. The increasing number of eligible patients, after the indication extension, has led to concerns about the cost-effectiveness and affordability of the treatment. Cost-effectiveness of adjuvant trastuzumab has been assessed in several studies, and in most cases it has been deemed as a cost-effective treatment [Citation13,Citation14]. However, most of these economic evaluations have been based on published interim results of clinical trials, having a relatively short follow-up. Evaluations using data from FinHer trial are few [Citation15–17], and there are no studies assessing the cost-effectiveness of short course trastuzumab in the light of the updated results.
The aim of this study was to assess the potential cost-effectiveness of adjuvant 9-week trastuzumab treatment, compared to treatment without trastuzumab, applying the final 5-year follow-up results of the FinHer trial [Citation11]. In addition, due to uncertainty related to the effect size of the 9-week treatment regimen and other model parameters, value of information (VOI) methods were used to combine the probability and consequences of a wrong adoption decision. Expected value of perfect information (EVPI) and expected value of perfect partial information (EVPPI) analyses were performed in order to address the question whether and what additional evidence is required to support an adoption decision.
Material and methods
A health-economic modelling approach was utilised in the study. The model was used to simulate a hypothetical cohort of 1000 HER2-positive patients of an average age of 50 years matching the inclusion criteria and baseline population characteristics of the FinHer trial [Citation11], the primary source of effectiveness data for this study. In the published results of the FinHer trial, the hazard ratio for distant disease progression was 0.65 (95% CI 0.38–1.12) and for death 0.55 (95% CI 0.27–1.11) after a follow-up of five years [Citation11]. Clinical parameters were extracted from published clinical trials. The analysis was performed from a societal perspective that included all direct, but no indirect costs (such as productivity losses). Costs and health outcomes were discounted by 3% as recommended in the Finnish guidelines. The evaluation model was built in Microsoft Excel 2007.
Model structure and clinical parameters
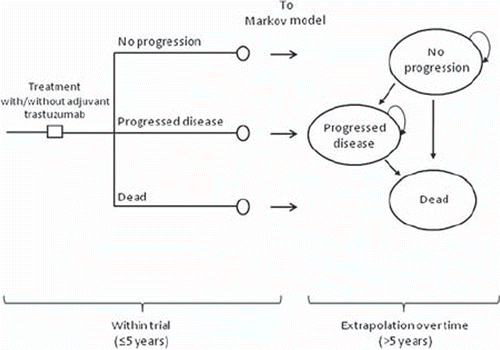
The applied model consists of two parts. The first part concerns the first five years from the initiation of treatment, and the second part continues onwards from year 5 to lifetime. This partition is driven by the available data on effectiveness, i.e. the actual survival curves, obtained from the final results of the FinHer trial [Citation11], which were used to inform the clinical outcomes of the first part of the model. The second part of the model is a traditional Markov stage-transition model [Citation18], which was used to extrapolate the costs and health outcomes over the lifetime of the patients. The initial Markov-stages (at the end of year 5) were populated using the information from model part 1. A scheme of the evaluation model is presented in .
Since the published Kaplan-Meier curves of overall survival (OS) and distant disease-free survival (DDFS) in the FinHer trial [Citation11] were not presented in a format suitable to health-economic analysis, a parametric (log-logistic) survival model was fitted to a manual trace of the curves, using statistical package R. The log-logistic model was chosen above other survival models (Weibull, exponential) because it provided the best fit to data (). Probabilities of being in one of the three health states at a certain point in time were derived from this model, together with the associated measures of uncertainty. The treatment effect of trastuzumab was estimated as a regression coefficient. Because neither the summaries of OS conditional on DDFS nor patient histories have been published, the conditional nature of the sequence of events (time from DDFS to OS) was imposed through a constraint in the simulation model.
Figure 2. Kaplan-Meier survival curves (continuous lines) and the fitted log-logistic model (dashed lines) for overall survival and distant disease-free survival for 9-week adjuvant trastuzumab. Vertical lines represent drop-outs. Original survival curves obtained from the FinHer trial [Citation11].
![Figure 2. Kaplan-Meier survival curves (continuous lines) and the fitted log-logistic model (dashed lines) for overall survival and distant disease-free survival for 9-week adjuvant trastuzumab. Vertical lines represent drop-outs. Original survival curves obtained from the FinHer trial [Citation11].](/cms/asset/7462e4e2-48c5-4580-ad13-0f62c6153826/ionc_a_553841_f0002_b.gif)
Reliable follow-up data from clinical trials were lacking from five years onwards, and thus a Markov model was populated with data on disease progression. Patients enter the Markov model (part 2 of the model) at the beginning of year 6 according to the proportions in each of the health states estimated in model part 1. The Markov model utilises monthly transition probabilities between health stages to represent the natural flow of the disease. The mutually exclusive health states are “No disease progression”, “Progressed disease”, and “Dead”. Only distant progressions are included in the definition of “Disease progression”. The risk of disease progression is assumed to gradually decrease over time, and no new distant progressions are assumed to occur after 20 years of disease-free survival. Distant disease progression is assumed to be treated with trastuzumab, regardless of initial treatment assignment. Transition probabilities utilised in the Markov-model are presented in . The transition probability from progressed disease to death was based on median overall survival (25.1 months) among patients with HER2+ metastatic breast cancer treated with trastuzumab [Citation19]. Disease progression and its treatment are modelled as tunnel states in order to allow variations in treatment length. Official Finnish life-tables, adjusted for breast cancer, were used to capture background mortality due to other causes than breast cancer [Citation20]. In the base case analysis, the treatment effectiveness of adjuvant trastuzumab was limited to five years, and beyond this point there were not assumed to be any differences in the treatment effectiveness between the compared groups (i.e. risk of disease progression was assumed to be same after five years in both groups). The modelled population contributes costs and outcomes each month according to the modelled health stages during the entire model presented in .
Table I. Model parameters and their probability distributions.
Quality of life parameters
Since appropriate Finnish quality of life data was not available, the applied utility weights were based on a study with 361 Swedish breast cancer patients [Citation21]. The utility weights () were measured with the EQ-5D quality of life instrument [www.euroqol.org]. The model calculates quality-adjusted life-years (QALY), which is a common outcome measure used in economic evaluations.
Cost parameters
Treatments of localised and disseminated cancer can be distinguished. Breast cancer-specific treatment costs (in 2008 Euros) were previously obtained from a Finnish university hospital [Citation22]. In addition, patient co-payments were added to the present analyses in order to be consistent with the study perspective. The aggregated per-cycle costs, used in the analysis, are presented in . Adjuvant trastuzumab is used in addition to standard breast cancer treatment, and it is given as an infusion. The short, 9-week, treatment protocol begins with a loading dose of 4 mg/kg and continues with a weekly 2 mg/kg. Upon disease progression, patients receive trastuzumab in 3-week cycles for a maximum of 52 weeks similarly in both groups. The costing includes acquisition, administration and preparation costs.
Sensitivity analyses
The model was made probabilistic in order to take into account the uncertainty related to all individual model parameters, and to convert this parameter uncertainty into decision uncertainty. In a probabilistic sensitivity analysis all model variables are allowed to vary simultaneously according to their probability distributions. Probability distributions were applied for all model inputs (probabilities, quality of life, costs). Beta-distributions, whose benefit is the unit interval (0 to 1), are applied for each of the probabilities and quality weights. The cost of trastuzumab was assumed to follow normal distribution because the dosing of trastuzumab is weight-related, and weight can be assumed to be normally distributed. Gamma-distributions were used for all other treatment costs. The fitted survival curves () were varied by drawing the correlated regression coefficients using Cholesky decomposition. With this method, the uncertainty in the clinical data can be explored without breaking the correlation structures imposed by the chosen parametric survival model (log-logistic in this case).
The model was calculated repeatedly for 1000 times in order to elicit the real variation of the results, rather than a single point estimate. The results of the simulation are presented in a cost-effectiveness plane. The uncertainty surrounding the cost-effectiveness of trastuzumab is depicted in a cost-effectiveness acceptability curve (CEAC), which plots the probability that trastuzumab is cost-effective for a range of cost-effectiveness thresholds.
In addition to the probabilistic sensitivity analysis, a variety of other sensitivity analyses were performed. In conventional one-way sensitivity analyses individual model parameters were altered one at a time. In one scenario, the assumption of trastuzumab being used after disease progression was tested by excluding the cost of trastuzumab treatment in advanced disease from the model. At the same time, the observed treatment benefit in advanced disease was also excluded, and the transition probability was based on median survival of 20.3 months [Citation19]. In another scenario, adjuvant trastuzumab was given for 12 months (€2800/month), while the treatment effect remained at the base-case level. In the final sensitivity scenario the first part of the model was removed, and the 3-stage Markov model (part 2) was used for the entire comparison. Here, the transitions based on fitted survival curves were replaced with constant transition probabilities (). With this modified model, we aimed to illustrate the uncertainty related to different assumptions of the effect of treatment benefit persisting beyond treatment duration.
Value of information analysis
The applied probabilistic model characterises the uncertainty related to the decision problem, and it was also used to establish the value of additional research aimed at obtaining more precise model parameters. Increased precision of the model parameters would reduce the decision uncertainty, i.e. minimise opportunity losses. In addition, value of additional research for a set of model parameters (i.e. clinical, quality of life, and cost parameters) was estimated to determine what type of additional research would be most valuable. Additional research may be considered worthwhile if the value of additional research in monetary units exceeds the cost of conducting such research.
Results
In the probabilistic base-case analysis, 9-week adjuvant trastuzumab treatment led to 0.66 incremental QALYs or 0.85 life-years gained (LYG) with an additional lifetime cost of €7900, compared to treatment without adjuvant trastuzumab. Thus, the incremental cost-effectiveness ratio (ICER) of the 9-week treatment was €12 000 per QALY, and €9300 per LYG. The total QALYs gained with and without adjuvant trastuzumab were 8.37 and 7.71, and the costs were €61 600 and €53 700, respectively. The probability that the 9-week course provides additional benefits at additional costs is high, but the hypothesis of minimal or even negative treatment effect cannot be excluded entirely (). Most (70%) of the 1000 iterations for incremental effectiveness lie between 0.3 and 1.0 QALY. Similarly, 70% of the estimates for incremental costs lie between €2900 and €12 300. According to these results, the short 9-week trastuzumab treatment is likely to be cost-effective already in relatively low willingness-to-pay (WTP) threshold levels. There is, for example, 87% probability of being a cost-effective option at WTP of €30 000 per QALY (). This figure also shows the observed variation of cost-effectiveness with chosen discount rate. The impact of the discount rate reflects the nature of the treatment, in a sense that health benefits in terms of survival will be received in distant future.
Figure 3. Incremental cost-effectiveness plane of adjuvant 9-week trastuzumab in early stage breast cancer in Finnish settings. QALY: Quality-adjusted life year.
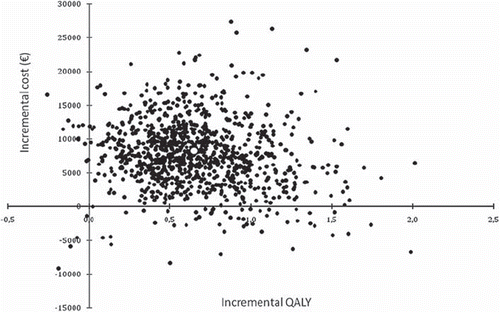
Figure 4. Cost-effectiveness acceptability curves (CEACs). (A) and expected value of perfect information (EVPI) (B) of adjuvant 9-week trastuzumab in early stage breast cancer in Finnish settings. Presented with discount rates 0%, 3% and 6% for future costs and outcomes.
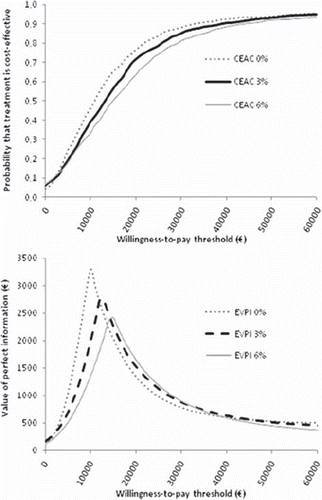
Value of information (VOI) analysis indicates that the patient-level expected value of perfect information (EVPI) is, for example, €870 at WTP of €30 000 per QALY (). The EVPI informs the consequences of making a wrong decision in monetary units, combined with the probability of that decision. With the WTP threshold of €30 000 and a population of 10 000 HER2-positive breast cancer patients, the population EVPI would be €8.7M. The EVPI is at its maximum at the point of ICER, where the decision uncertainty is greatest. The expected value of perfect partial information (EVPPI) showed that more than 90% of this can be attributed to the effectiveness parameters.
Sensitivity analyses
In the deterministic one-way sensitivity analyses the model parameters were altered one at a time. The results were relatively sensitive to the alteration of discount rate and trastuzumab treatment costs. Most parameter uncertainty, however, was related to the treatment effectiveness of adjuvant trastuzumab. When the bounds of 95% confidence interval of treatment co-efficient during years 0–5 were used, the ICER varied from €2 500/QALY to being dominated. The inclusion of costs of cardiac monitoring or additional travel costs did not have any significant impact on the results.
Assumptions related to trastuzumab use in advanced disease were tested in a scenario, where trastuzumab was discarded from treatment of advanced disease in both groups. This had only a marginal effect on the cost-effectiveness results, largely due to the fact that both groups were assumed to be treated similarly after disease progression. The impact of treatment length was studied in another scenario. When trastuzumab was used according to the 12-month treatment schedule, the ICER was €49 600 per QALY, assuming that the treatment benefit was the same as in the base case.
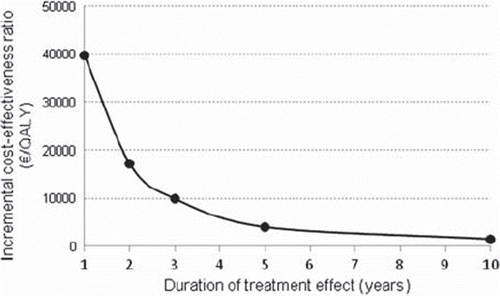
Another important aspect related to the treatment effect is the assumption of the length of clinical benefit received beyond the duration of treatment. Our cost-effectiveness model utilised fitted survival data, and thus no further assumptions of the carry-over time of treatment effect were needed. However, to demonstrate how assumptions related to the length of treatment effect could affect the cost-effectiveness results, model part 1 was replaced with constant transition probabilities for disease progression (0.0031/0.0048 per cycle). These probabilities were applied for different durations, as illustrated in . There is a strong dependence with the assumed length of treatment effect and cost-effectiveness of the treatment. Thus, it is clear that a conclusion on a treatment's cost-effectiveness may depend on the assumptions related to carry-over time of treatment benefit.
Discussion
The aim of the current study was to assess the potential cost-effectiveness of adjuvant 9-week trastuzumab treatment in Finnish settings using the final results of FinHer trial, which have not, to our knowledge, been previously utilised in economic evaluations. We found that 9-week adjuvant trastuzumab is likely to be a cost-effective treatment option in early breast cancer compared to treatment without trastuzumab, despite the uncertainty related to the treatment effect. The value of information analyses show that more investment should be directed especially to research related to treatment effect in order to reduce the uncertainty related to adoption decision. The maximum acceptable cost (population EVPI) for a trial that would eliminate all uncertainty depends on the number of patients and society's willingness-to-pay threshold level.
The first published results from the clinical trials concerning adjuvant trastuzumab treatment were with relatively short follow-up (from 1 to 3 years) [Citation6–8,Citation10]. The more recently updated results show that the treatment effect of trastuzumab may be less favourable as previously expected. In the Herceptin Adjuvant (HERA)–trial, using 12-month regimen, the hazard ratio (HR) for the risk of an event increased from 0.54 to 0.64 as the follow-up period changed from one year to two years [Citation6,Citation9]. The updated results of FinHer trial show similar change with the 9-week treatment. After 3-year follow-up, the women treated with 9-week trastuzumab + chemotherapy had more favourable overall survival (OS) compared to those treated with chemotherapy alone (Hazard Ratio [HR] = 0.41; 95% CI 0.16 to 1.08) [Citation10]. However, after a 5-year follow up, the corresponding HR was 0.55 (95% CI 0.27 to 1.11) [Citation11]. In the FinHer trial, the patient subgroup with HER2 over-expression was small (n = 232) compared to the other studies. Despite of the size of the patient population, the short course adjuvant treatment has shown positive signs about its efficacy, compared to chemotherapy only. Nevertheless, the level of statistical significance, related to survival estimates, needs to be considered. In the current study adjuvant trastuzumab led to 0.66 additional QALYs or 0.85 life-years. If our analysis would have been based on subgroup that received docetaxel+FEC+trastuzumab, instead of any chemotherapy+trastuzumab, the results would be more beneficial to trastuzumab. However, this was not used since it does not reflect the current treatment practice, and also the population would have been considerably smaller. In addition, no patient subgroup analyses were performed due to lack of adequate information and sample size. The potential impact of patient cross-over from control group to receiving active treatment has not been addressed in the current study.
Economic evaluations are being used increasingly often in attempt to meet the challenges of optimally allocating the scarce health care resources. Cancer presents a challenge to health care funding both due to more expensive treatments and rising incidence rates. Furthermore, due to ageing of the population the annual number of new cases is increasing more rapidly than the age adjusted incidence. When expensive drugs are used in a number of patients, the economic issues become even more important. Trastuzumab has been available for metastatic BC from year 2000, and in 2006 the indication was extended for the adjuvant treatment of HER2-positive early breast cancer. This indication extension multiplied the number of potential patients. Since then, the cost-effectiveness of adjuvant trastuzumab has been investigated in several publications, and it has been estimated to be cost-effective in most of the analyses. However, a large part of these analyses are based on interim results of efficacy, and thus may need to be updated. Results from cost-effectiveness analyses based on the one-year HERA results [Citation6] have varied from €6000 per QALY to £18 000 (€22 000) per QALY [Citation14]. Analyses based on the 2-year HERA results [Citation9] have presented results from €17 000 per LYG to 127 900 Canadian dollars (€96 000) per QALY [Citation14]. From UK perspective, an ICER of £25 803 (€31 600) per QALY has been recently reported when 2-year follow-up was included in the analysis [Citation24]. Based on the above mentioned results, it seems these cost-effectiveness results are strongly associated with the follow-up time in the original study. However, the analyses based on shorter follow-up currently outnumber those using longer follow-up. A similar phenomenon may now be detected from economic evaluations based on 9-week treatment. In an Australian study, 9-week treatment had an ICER of A$1700 (€1300) per QALY [Citation16], and in a Swiss study it was found to be cost-saving [Citation15]. A Belgian study showed that 9-week adjuvant treatment is most of the times cost-saving (in 11 of 15 subgroups), and has ICER above €30 000/LYG in only one of the patient subgroups (age 80 + , stage I disease) [Citation17]. In the present analysis, we found a very low probability of 9-week trastuzumab being cost-saving, though the ICER was still on an acceptable level.
Trastuzumab use and its economic consequences have been studies in Nordic countries in recent years. In Norway, the incremental cost per life-year saved ranged between €8148 and €35 947 for a 1-year adjuvant treatment. The study assumed 10% or 20% improvement in absolute overall survival with trastuzumab treatment [Citation25]. The actual use of trastuzumab has been studied in Swedish Health Care Regions [Citation26], and from Swedish societal perspective the ICER for 1-year adjuvant trastuzumab was estimated to be €36 000 or €41 500 per QALY, in base-case analysis, depending on the HER2-testing strategy [Citation27].
The current study was based on modelling, which inherently leads to simplification of real life circumstances. The model structure was driven by the available information in the primary data source. In the utilised Markov model (part 2), three mutually exclusive health stages were used, following the partition in model part 1. Since the analysis was based on published results of the FinHer trial, the inclusion of additional health stages would have led to number of assumptions leading to unnecessary uncertainty. For example, the local recurrences could not have been reliably included in the model, since little information has been published on this in the original data source [Citation11]. Moreover, distant recurrences are more closely associated with mortality than local ones [Citation11]. Nevertheless, the modelled health outcomes in the present study were of a same magnitude with those reported in previous studied assessing adjuvant trastuzumab. In studies based on 12-month treatment, the incremental life-years gained with trastuzumab ranged from 0.12 [Citation15] to 4.1 [Citation16] years depending on the timeline and discount rate. Similarly, in studies using the interim results of FinHer trial [Citation10] the incremental life-years ranged from 0.27 [Citation15] to 5.9 years [Citation16]. If subgroup analyses were taken into account (e.g. age and disease stage) the variation in additional health benefit would be larger than the above mentioned [Citation17]. The utilised cost data was based on a previous Finnish study [Citation22]. When compared with other economic evaluations of adjuvant trastuzumab, the stage specific costs were of similar magnitude to those used by others. Cost of HER2-testing was not included in the analysis since all new patients are equally tested. Similarly, cardiac toxicity was not taken into account in the analysis, because in the FinHer trial no significant differences existed between patients treated with or without trastuzumab [Citation11]. In addition, relatively fewer cases with cardiac toxicity were observed during the short course treatment in FinHer [Citation11], compared to trials using 12-month treatment [Citation28].
Quality of life was based on Swedish breast cancer patients [Citation21], because suitable information was not available from local patients. However, due to close geographical and cultural proximity of Finland and Sweden, we believe that the utilised values were adequate to be used in the analyses.
In a sensitivity scenario we illustrated how different assumptions may affect the result of cost-effectiveness analyses. This is a key tool to determine the impact of various assumptions on the results, and is essential in complicated models, where the individual impact of each assumption on the observed results is difficult to infer analytically. Similar observations regarding the impact of assumed length of treatment benefit have been reported by others [Citation24]. In previously published cost-effectiveness analyses, five years has been the most used duration of benefit [Citation14]. In the current study we applied information directly from the published survival curves for OS and DDFS, which were used to estimate the natural flow of the disease for the first five years. Thus, we demonstrated that, despite their drawbacks, published Kaplan-Meier curves can be an appropriate source for fitted survival data in economic evaluations. Because of the statistical non-significance of the difference in treatment effects, our model included the possibility that adjuvant trastuzumab would lead to worse outcomes than the comparator. However, eventually only a small minority of the modelled cases showed negative effect.
Economic issues related to cancer treatments are multifaceted and even politically sensitive due to equity issues and the social value of the disease [Citation29]. The Pharmaceutical Management Agency of New Zealand (PHARMAC) restricted trastuzumab funding to cover only 9-week of treatment, in July 2007 [Citation30]. However, this turned into a juridical and political issue, and eventually the funding was extended to include 12-month treatment [Citation31,Citation32]. There is currently no evidence comparing the efficacy of the 9-week and 12-month treatment regimens, and thus the relative efficacy of these treatments cannot be evaluated directly. With the currently available data, economic evaluations based on an indirect comparison of these treatments would support the 9-week treatment. However, with the current level of information and uncertainty, such evaluations would not be sufficiently credible to aid decision-making. The 9-week treatment may be a promising option in economic terms, compared with the 12-month treatment, if the treatment effect observed in existing studies is confirmed in additional studies. The ongoing SOLD-trial [Citation12] will eventually provide an important answer to this question. Since the duration of the treatment benefit is one of the driving forces of cost-effectiveness, such studies with longer follow-up are needed for more precise evaluations. Economic analyses on adjuvant trastuzumab should be updated when new data is available.
In conclusion, the current study shows that 9-week adjuvant trastuzumab is likely to be cost-effective in Finnish setting at relatively low willingness to pay threshold levels. The sensitivity analyses and value of information analysis show that more research should be focused on the long-term effectiveness of treatments.
Acknowledgements
The study was supported in part by research grants from Yrjö Jahnsson foundation and Pharma Industry Finland research trust. All decisions related to the study were made on the basis of scientific issues with no editorial interference from the sponsors. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Finnish Cancer Registry [Internet]. Helsinki: Institute for Statistical and Epidemiological Cancer Research; 2010. [cited 2010 Oct 2]. Available from: http://www.cancerregistry.fi
- Mäklin S, Rissanen P. Cost of cancer – Treatment and productivity costs in 1996–2004 and forecast to year 2015. Finnish Cancer Organizations Publication no. 67. Helsinki, Finland: Finnish Cancer Organization; 2006. Finnish.
- Joensuu H, Isola J, Lundin M, Salminen T, Holli K, Kataja V, . Amplification of erbB2 and erbB2 expression are superior to estrogen receptor status as risk factors for distant recurrence in pT1N0M0 breast cancer: A nationwide population based study. Clin Cancer Res 2003;9:923–30.
- Owens MA, Horten BC, Da Silva MM. Her2 amplification ratios by fluorescence in situ hybridization and correlation with immunohistochemisty in a cohort of 6556 breast cancer tissues. Clin Breast Cancer 2004;5:63–9.
- Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, . Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 1989;244:707–12.
- Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, . Herceptin Adjuvant (HERA) Trial Study Team. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 2005;353:1659–72.
- Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE Jr, Davidson NE, . Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 2005;353:1673–84.
- Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Rolski J, . Phase III randomized trial comparing doxorubicin and cyclophosphamide followed by docetaxel (AC→T) with doxorubicin and cyclophosphamide followed by docetaxel and trastuzumab (AC→TH) with docetaxel, carboplatin and trastuzumab (TCH) in Her2neu positive early breast cancer patients: BCIRG 006 Study (abstract 62). Cancer Res 2009;69:500s.
- Smith I, Procter M, Gelber RD, Guillaume S, Feyereislova A, Dowsett M, . HERA study team. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: A randomised controlled trial. Lancet 2007; 369(9555):29–36.
- Joensuu H, Kellokumpu-Lehtinen PL, Bono P, Alanko T, Kataja V, Asola R, . FinHer Study Investigators. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med 2006;354:809–20.
- Joensuu H, Bono P, Kataja V, Alanko T, Kokko R, Asola R, . Fluorouracil, epirubicin, and cyclophosphamide with either docetaxel or vinorelbine, with or without trastuzumab, as adjuvant treatments of breast cancer: Final results of the FinHer Trial. J Clin Oncol 2009;27:5685–92.
- The Synergism Or Long Duration (SOLD) Study. ClinicalTrials.gov Identifier: NCT00593697.
- Chan AL, Leung HW, Lu CL, Lin SJ. Cost-effectiveness of trastuzumab as adjuvant therapy for early breast cancer: A systematic review. Ann Pharmacother 2009;43:296–303.
- Reed SD, Schulman KA. Cost utility of sequential adjuvant trastuzumab for HER2/Neu-positive breast cancer. Value Health 2009;12:637–40.
- Dedes KJ, Szucs TD, Imesch P, Fedier A, Fehr MK, Fink D. Cost-effectiveness of trastuzumab in the adjuvant treatment of early breast cancer: A model-based analysis of the HERA and FinHer trial. Ann Oncol 2007;18:1493–9.
- Millar JA, Millward MJ. Cost effectiveness of trastuzumab in the adjuvant treatment of early breast cancer: A lifetime model. Pharmacoeconomics 2007;25:429–42.
- Neyt M, Huybrechts M, Hulstaert F, Vrijens F, Ramaekers D. Trastuzumab in early stage breast cancer: A cost-effectiveness analysis for Belgium. Health Policy 2008;87:146–59.
- Briggs A, Sculpher M. An introduction to Markov modelling for economic evaluation. Pharmacoeconomics 1998;13:397–409.
- Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, . Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344:783–92.
- Statistics Finland. Statistical yearbook of Finland 2008 – The official statistics of Finland. Helsinki: Statistics Finland; 2008.
- Lidgren M, Wilking N, Jönsson B, Rehnberg C. Health related quality of life in different states of breast cancer. Qual Life Res 2007;16:1073–81.
- Purmonen T, Auvinen PK, Martikainen JA. Budget impact analysis of trastuzumab in early breast cancer: A hospital district perspective. Int J Technol Assess Health Care 2010;26:163–9.
- Clarke M, Collins R, Darby S, Davies C, Evans E, Godwin J. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet 2005;365(9472):1687–717.
- Hall PS, Hulme C, McCabe C, Oluboyede Y, Round J, Cameron DA. Updated cost-effectiveness analysis of trastuzumab for early breast cancer: A UK perspective considering long-term toxicity and pattern of recurrence. Leeds Institute of Health Sciences, Working Paper. June 2010.
- Norum J, Olsen JA, Wist EA, Lønning PE. Trastuzumab in adjuvant breast cancer therapy. A model based cost-effectiveness analysis. Acta Oncol 2007;46:153–64.
- Wilking U, Jönsson B, Wilking N, Bergh J. Trastuzumab use in breast cancer patients in the six Health Care Regions in Sweden. Acta Oncol 2010;49:844–50.
- Lidgren M, Jönsson B, Rehnberg C, Willking N, Bergh J. Cost-effectiveness of HER2 testing and 1-year adjuvant trastuzumab therapy for early breast cancer. Ann Oncol 2008;19:487–95.
- Telli ML, Hunt SA, Carlson RW, Guardino AE. Trastuzumab-related cardiotoxicity: Calling into question the concept of reversibility. J Clin Oncol 2007;25:3525–33.
- Drummond M, Evans B, LeLorier J, Karakiewicz P, Martin D, Tugwell P, . Evidence and values: Requirements for public reimbursement of drugs for rare diseases – a case study in oncology. Can J Clin Pharmacol 2009;16:e273–81.
- Metcalfe S, Evans J, Priest G. PHARMAC funding of 9-week concurrent trastuzumab (Herceptin) for HER2-positive early breast cancer. N Z Med J 2007;120(1256):U2593.
- Herceptin now an election issue. [cited 2010 Nov 2]. Available from: http://www.stuff.co.nz/national/herceptin-debate/569826/Herceptin-now-an-election-issue
- The Pharmaceutical Management Agency of New Zealand [Internet]. [updated 2011 Jan 26; cited 2010 Nov 2]. Wellington: PHARMAC; 2008. Available from: http://www.pharmac.govt.nz/herceptin#gov