Abstract
The current paper presents Chapter 5 of the second edition of the European Guidelines for Quality Assurance in Cervical Cancer Screening, which deals with the histopathological diagnosis of lesions of the uterine cervix. It completes a series of publications in journals containing the contents of other parts of the European Guidelines. Histopathology provides the final diagnosis on the basis of which treatment is planned, and serves as the gold standard for quality control of cytology and colposcopy. It is also the source of the diagnostic data stored at the cancer registry and used for evaluation of screening programmes. It is therefore important that histopathology standards are monitored and based on agreed diagnostic criteria. Histology is required to diagnose the degree of abnormality in women with persistent low-grade abnormalities including HPV-lesions, as well as high-grade lesions. Cytology may also suggest either glandular abnormalities or be suggestive of high-grade CIN, AIS or invasive cancer. Histopathologists should be aware of, and familiar with, the nature of cytological changes which may be relevant to their reports. The accuracy of the histopathological diagnosis of tissue specimens depends on adequate samples, obtained by colposcopically directed punch biopsies (with endocervical curettage if necessary) or excision of the transformation zone or conisation. An accurate histological diagnosis further depends on appropriate macroscopic description, technical processing, microscopic interpretation and quality management correlating cytological and histological diagnosis. This paper proposes guidelines for sampling and processing of cervical tissue specimens obtained by biopsy, excision and/or curettage.
The current paper presents Chapter 5 of the second edition of the European Guidelines for Quality Assurance in Cervical Cancer Screening, which deals with the histopathological diagnosis of lesions of the uterine cervix. It completes a series of publications in journals containing the contents of other parts of the European Guidelines [Citation1–7].
Cervical cytology currently represents the primary screening method, but does not provide the final diagnosis. Abnormal cervical cytology should be followed by colposcopy and microscopic evaluation of cervical tissue [Citation8].
Adequate colposcopy is neccessary to locate the most abnormal areas of the cervix [Citation9]. The criteria for colposcopic referal and the requirements for high-quality colposcopy were described in Chapter 6 of the European Guidelines for Qualtity Assurance of Cervical Cancer Screening [Citation7,Citation10,Citation11]. The validity of the histopathological report will also depend on the quality of the biopsy. Since these specimens are often very small (in the range of millimeters), careful handling and work-up is required.
If positive cytology does not correlate with the histological findings from the biopsies, the pathologist has to consider that a dysplastic lesion could be small and missed by the biopsy or alternatively not visible due to endocervical localisation. For this reason histology and cytology should be closely correlated to give the gynaecologist a clear impression of the individual situation.
Excision biopsy represents a special type of tissue specimen. Its objective is the complete removal of dysplastic lesions, found by a previous biopsy and/or cytology. The histopathological report of an exision biopsy should include a clear diagnosis of the primary lesion and a description of the resection margins. Since possible micro-invasion has a major impact on the management of patients, complete work-up of excised tissue in step serial sections is recommended. Additional immunohistochemistry in selected cases might support a diagnosis of possible microinvasion or vessel involvement and might help in the distinction between squamous or glandular neoplasia [Citation12,Citation13].
1The presented recommendations are mainly based on existing practice and experience of European experts in gynaecological histopathology. It is the intention of the ECCG Network*, who will prepare updated future guidelines, to assess the quality and level of evidence related to alternative procedures in cervical histopathology, in particular on the role of new molecular markers, following procedures of evidence-based medicine.
Punch biopsies
Punch biopsies are small pieces of tissue a few millimeters in diameter that are removed from the cervical mucosa with a biopsy forceps. For indications: see Chapter 6 of the EU guidelines [Citation6] .
Diagnostic goal
When colposcopy is satisfactory and obvious area(s) of CIN can be visualised, histological examination of punch biopsies can be sufficient to obtain a correct diagnosis.
Macroscopic description
The number, diameter, colour and consistency of the specimens should be documented.
Technique
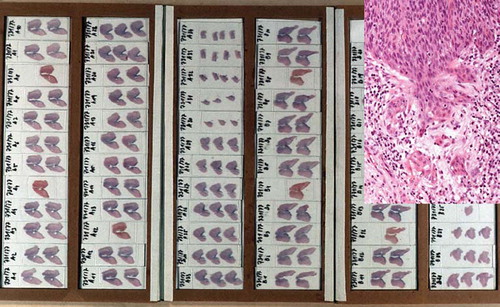
In case of multiple cervical biopsies, each area of the cervix from which the biopsies have been taken should be identified seperately. Specimens are fixed in 4% buffered formalin at room temperature, followed by paraffin embedding according to routine procedures. Four micrometre paraffin serial tissue sections are stained for H&E and/or processed for special stains and immunohistochemistry, if indicated.
Histological diagnosis
The histological report should include:
Tissue type
Absence or presence and type of neoplastic lesions
Grade of identified lesions2:
Squamous lesions: cervical intraepithelial neoplasia 1–3 (CIN1-3), invasive cancer.
Glandular lesions: high-grade and low-grade cervical glandular intraepithelial neoplasia (CGIN), invasive adenocarcinoma or adenosquamous carcinoma.
Presence of HPV-associated changes (koilocytes, dyskeratosis)
Size of the lesion (mm)
Characterisation of non-neoplastic lesions
Stromal reaction: presence and extent of inflammation or desmoplastic reaction
In case of invasive cancer, depth and lateral extent of invasion, presence of lymphovascular involvement and the degree of differentiation should be documented
These guidelines strongly recommend the CIN classification for histological diagnosis. Careful attention to criteria for diagnosis of the three grades of CIN (CIN1-3) should be observed [Citation15]. CIS is usually combined with CIN3 (in the UK both are recorded as “in-situ carcinoma of the uterine cervix” in the national cancer registry).
Grading of CIN reflects biology of the underlying lesion. Broadly speaking, CIN1/koilocytosis (correlating to LSIL) is likely to be reversible and associated with productive HPV infection [Citation16]. CIN2 and CIN3/carcinoma in situ (correlating to HSIL, Bethesda classification) are more likely to persist or progress if left untreated and also more likely to be associated with HPV integrated into the host genome [Citation16]. Two meta-analyses of follow-up studies indicate a greater likelihood of regression and a lesser likelihood of progression with CIN2 compared with CIN3 [Citation17,Citation18]. CIN3 is a more robust diagnosis than CIN2 and is therefore more useful as a gold standard for outcome [Citation19].
In small biopsies it may occasionally be necessary to report CIN as “ungraded” but where possible diagnoses such as CIN1-2 should be avoided.
The distinction between individual grades of CIN is poorly reproducible but improves with increasing grade. Diagnoses of CIN3 and invasive cancer are the most reproducible [Citation20,Citation21]. Immature squamous metaplasia and atrophic squamous epithelium are documented sources of misinterpretation and may be mistaken for CIN1-2 [Citation22]. In such cases p16 staining and repeat biopsy after oestrogen may be helpful [Citation23] (see also section on immunohistochemistry, below). In small biopsies it may occasionally be necessary to report CIN as “ungraded” but where possible diagnoses such as CIN1-2 should be avoided.
Precise grading of CGIN is poorly reproducible and there is little evidence that it forms a biological spectrum [Citation15]3.
High-grade CGIN equates to adenocarcinoma in situ and low-grade CGIN is usually managed in the same way. Low-grade CGIN should be reported infrequently and care must be taken to distinguish it from benign conditions that may mimic it [Citation24]. The same strictures apply to diagnoses of glandular dysplasia and atypia [Citation15,Citation25].
Excision biopsies
Excision biopsies represent nearly cone-shaped portions of cervical tissue including the lower part of the endocervical canal and a portion of the ectocervix. Excision biopsies include cold knife conisation, laser conisation and Large Loop Excision of the Transformation Zone (LLETZ)4.
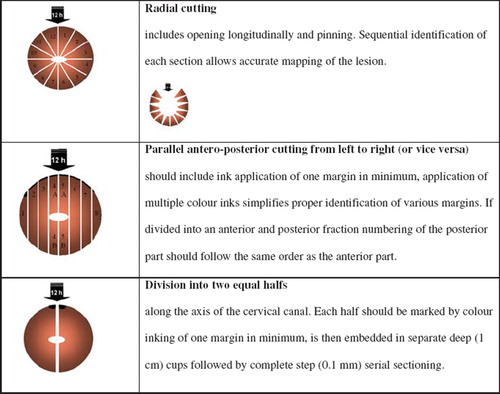
Cold knife (and laser) cone biopsies are indeed cone shaped tissue specimens whereas LLETZ excisions in most cases represent a more disc shaped, ectocervical portion sometimes with an extra biopsy from the middle of the endocervical canal (top hats, Mexican hats). The histopathologist should be able to recognise and deal with these different forms of excision biopsies. For technical details of excision and clinical indications, see Chapter 6 of the EU guidelines [Citation6].
Diagnostic goals
An excision biopsy should aim to remove all pathological tissue (identified by colposcopy) including a part of the endocervical canal and the transformation zone. The procedure should be diagnostic (provide a precise histological diagnosis) and therapeutic (resection of the lesion in toto).
Macroscopic description
Description should include the size of the specimen (length and diameter), localisation of the cervical canal (central, paracentral or marginal), any visible lesion, and the position of any markings and sutures for orientation of the specimen [Citation26].
Technique
Usually an exision biopsy removes the whole transformation zone, including a portion of the lower endocervical canal.
The biopsy should be marked clearly (e.g. colour or threads at 12 o'clock) to enable adequate orientation throughout the future workup [Citation27,Citation28]. The integrity of the cervical canal should be preserved and not altered by prior dilatation.
There exist various techniques for sectioning excision biopsies [Citation29]. The methods used include opening, pinning and serially sectioning the specimen – or fixing and serially sectioning the unopened specimen at right angles to the os. A simple and easily reproducible method is the division of the tissue into two equal halves along the axis of the cervical canal. Each half is embedded in-separate deep (1 cm) cups followed by complete step (0.1 mm) serial sectioning. This method is described in the guidelines of the Austrian Society of Pathology [Citation30] and results in histological slides that are easy to orient and interpret, including inmost cases an accurate evaluation of the resection margins ( and ).
Histological diagnosis
Histological reports on an excision biopsy should provide a well defined pathological diagnosis as summarised below. The diagnosis should also be in concordance with the WHO histological classification of tumours of the uterine cervix ( and ). In addition to a precise description of the histological type of the lesion the report should include information concerning the:
Grade of neoplastic lesion
Localisation of the lesion within the excision biopsy
Uni/multifocality of the lesion
Extent of the lesion (in case of microinvasive and invasive cancer), measurement of vertical and horizontal diameters is crucial for adequate staging
Stromal reaction
Involvement of microvessels
Relation of tumour tissue to all resection margins (distance)
Description and characterisation of additional non-neoplastic lesions (tuboendometroid metaplasia, microglandular hyperplasia, endometriosis, regenerative- and repair changes)
Table I. Histological classifications of preinvasive intraepithelial lesions of the uterine cervix.
Table II. WHO histological classification of malignant tumours of the uterine cervix [Citation15].
The term microinvasive carcinoma may be applied to squamous cell carcinomas and adenocarcinomas but only when accompanied by measurements of depth and lateral extent of a completely excised lesion. The diagnosis can then be defined according to the FIGO definitions of stage 1A1 and 1A2 ( and ), for which there is an evidence base for outcome after treatment [Citation15]. Depth of invasion should be measured from the base of the epithelium from which the invasive lesion arises and the lateral extent from the section in which the width is widest. Stage 1A1 lesions (less than 3 mm depth and less than 7 mm width) should be specified as either one or more foci of early stromal invasion or a confluent lesion. Stage 1A2 lesions are defined as 3–5 mm depth and less than 7 mm width.
Table III. TNM categories and FIGO staging.
Figure 3. Staging of cervical cancer according to FIGO (Montreal, 1994) extracted from [Citation44].
![Figure 3. Staging of cervical cancer according to FIGO (Montreal, 1994) extracted from [Citation44].](/cms/asset/42dd551e-ea2c-41e5-b182-30bde33f371b/ionc_a_555779_f0003_b.jpg)
Adenocarcinomas should be measured and recorded in the same way but there are no reliable criteria for distinguishing 1A1 and 1A2 tumours.
If an invasive lesion cannot be measured as indicated above, it should be described as a small invasive carcinoma and classified as 1B1. The presence of lymphovascular invasion should be recorded but does not affect the FIGO stage.
Endocervical curettage (ECC)
Endocervical curettage (ECC) is a sampling procedure to obtain endocervical tissue. Readers are refered to Chapter 6 of the EU guidelines [Citation6] for the clinical indications of ECC.
Diagnostic goal
The objectives of ECC are:
to evaluate any ectocervical squamous cell lesion extending to the endocervical canal
to detect endocervical adenocarcinoma and its precursor lesions
to determine cervical involvement of any non-cervical malignancies
Endocervical curettage combined with colposcopically directed ectocervical punch biopsies allows histological assessment of both the ecto- and endocervix, without excising a substantial amount of cervical tissue. Nevertheless, it should be kept in mind that ECC has a limited sensitivity to detect endocervical CIN or CGIN. Furthermore, ECC alters the architecture of the endocervical canal, compromising the assessment of a later conisation. Collection of endocervical cells, using an endocervical brush, has in several studies shown a higher sensitivity (but a lower specificity) then ECC [Citation31–34]. Other authors support the use of ECC, since it allows the detection of colposcopically hidden lesions [Citation35].
Macroscopic description
The number, diameter, colour and consistency of the specimen fragments should be documented.
Technique
ECC provides tissue from the endocervical canal by using an endocervical curette. Tissue from all four sides of the cervical canal should be obtained. Very small specimens should be wrapped in paper prior to paraffin embedding.
Serial sections of the biopsy specimens are recommended.
Histological diagnosis
The description of tissues found in the curetted material should specify:
The presense of endocervical glands, endometrial tissue, squamous epithelium
Glandular or squamous intraepithelial neoplastic and non-neoplastic changes
Evidence for invasion
Neoplastic or non-neoplastic stromal alterations, and
Presence and kind of inflammatory processes
Immunohistochemistry
Immunhistochemistry might be helpful, if H&E stained sections do not provide enough information for inclusion or exclusion of intraepithelial or invasive neoplasia. Immunohistochemical staining of dysplastic lesions of the cervix with a variety of antibodies to cell cycle-associated proteins can provide additional information in those difficult cases.
Proliferation markers are widely used by pathologists and can be easily applied on formaline fixed and routinely processed cervical tissues.
The Ki-67 antigen is a non-histone protein expressed in the nucleus in all phases of the cell cycle except G0. The most commonly used monoclonal antibody for immunohistochemical detection of the Ki-67 antigen in paraffin sections is clone MIB1. The extent of Ki-67 immunostaining generally parallels increasing grades of dysplasia [Citation36]. Moreover, expression of Ki-67 allows distinction of atrophic cervical epithelium (negative for Ki-67) from neoplastic or dysplastic cervical epithelium (positive for Ki-67) [Citation37].
The proliferating cell nuclear antigen (PCNA) has been identified as a polymerase-associated protein and is synthesised in early G1 and S phases of the cell cycle and might be also helpful [Citation38].
Cervical neoplasia, but not other cervical epithelia, expresses high levels of the cyclin-dependent kinase inhibitor p16, suggesting that staining for this marker could provide diagnostic support to distinguish true CIN/dysplasia from immature metaplasia or other non-neoplastic changes of the cervix. Immuno-detection of p16 in dysplastic epithelium using monoclonal antobodies in routinely processed histological cervical tissue was recently described by Klaes et al [Citation39].
Other immunohistochemical markers like antibodies directed to extracellular matrix components of the basal membrane could be used for the assessment of possible microinvasion in selected cases. Several studies have shown that routine H&E slides are not always adequate for detection of vascular invasion, especially in cases with strong inflammatory stromal reaction. Antibodies against endothelial marker proteins, e.g. Factor VIII-related antigen, stain both lymphatic and blood vessel endothelium and therefore represent a useful tool for the detection of lymphovascular invasion in cervical cancer. For a more selective assessment of blood vessels, CD31 can be recommended. For detection of lymph vessel involvement, immunostaining with newly recognised lymphendothelial proteins (like podoplanin) can be performed [Citation12,Citation13].
Data collection
Laboratories should provide a standard request form for collaborating gynaecologists including administrative patient data, previous reports of cytology, colposcopy, and cervical/uterine/vaginal/vulva histology. Indication for the intervention and the type of biopsy (punch, LEEP/LLETZ, cone, ECC, endocervical brushing) must be stated clearly.
Computerised documentation of histological reports and adequate storage of parafin blocks and sections (slides) must follow the local legal requirements for data protection. Often blocks and slides are kept indefinitely, the principle being to hold them for at least the life time of the patient.
As a minimum data should include:
patients’ key data
date of request
specification of material, and
a detailed and summarising histological report, coded, following a recognised international standard for histological classification (such as SNOMED, CIN/CGIN)
Histological data should be communicated to the national or regional screening register in order to correlate data as explained in Chapter 2 of the EU guidelines [Citation40]. Linkage of histological outcomes with screening histories, within the laboratory or in collaboration with the screening register, should allow the creation of cyto-histological cross tables and assessment of the predicitve value of cytology (see Chapter 7 of the EU guidelines [Citation41]).
Archived Pap smears and histological blocks of cervical tissue constitute a very important source for bio-bank research. The EU is currently promoting systems allowing high-quality research using stored human biological material (http://www.cancerbiobank.org/).
Quality assurance
All personnel involved in the histological part of the cervical screening process should understand each step of the entire work up procedure. Internal process-oriented quality assessment should include a laboratory handbook, safety instructions and protocols [Citation42,Citation43]. Histological reports should allow comparison and correlation with cytology and colposcopy.
Regular internal meetings for technical troubleshooting, training and diagnostic discussion should complete the working procedure. Additionally, interdisciplinary meetings of pathologists, cyto-technicians and gynaecologists with discussion of cytological slides, colposcopic images and histological slides are recommended.
The readers are referred to Chapter 4 of the EU guidelines for details concerning continuing education and external quality control in cytopathology [Citation43].
Acknowledgements
The financial support of the European Commission through the European Cervical Cancer Screening Network and the European Cancer Network is gratefully acknowledged. The content of this article is derived from the European Guidelines for Quality Assurance in Cervical Cancer Screening©, European Commission, 2008. The full document can be downloaded from: http://bookshop.europa.eu/eubookshop/download.action?fileName=ND7007117ENC_002. pdf&eubphfUid=10027853&catalogNbr=ND-70-07-117-EN-C.
The views expressed in this article are those of the authors and do not necessarily reflect the official position of the European Commission.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Notes
1The last two sentences are added to the printed book of the 2nd Edition of the European guidelines on Quality Assurance in Cervical Cancer Screening (Office for Official Publications of the European Communities, Luxembourg, 2008, editors: Arbyn M et al).
*ECCG: European Cooperation on Development and Implementation of Cancer Screening and Prevention Guidelines.
2CIN1 (flat condyloma; koilocytosis; mild dysplasia): Neoplastic, basaloid cells and mitotic figures occupy the lower third of the epithelium in CIN1 lesions. These lesions frequently show marked HPV cytopathic effects including perinuclear halos, multinucleation and nuclear membrane irregularities, and hyperchromasia (e.g., “koilocytosis”).
CIN2 (moderate dysplasia): In CIN2, neoplastic basaloid cells and mitotic figures occupy the lower two thirds of the epithelium.
CIN3 (severe dysplasia lumped with carcinoma in situ): The characteristic histological feature of CIN3 is the presence of neoplastic basaloid cells and mitotic figures that occupy the full thickness of the epithelium. These cells have high nuclear:cytoplasmic ratios, with scant cytoplasm and dense, hyperchromatic nuclei having coarse clumped chromatin and irregular nuclear outlines [Citation14].
3CGIN is recognised histologically by a combination of architectural and cytological abnormalities, though a consistent feature is the presence of nuclear abnormalities. Not all features are seen in every case. Architectural features include glandular crowding, branching and budding; intraluminal papillary projections; cribriform pattern. Cytological features include abrupt junction between normal and abnormal epithelium; intestinal/goblet cell metaplasia; loss of mucin-secretion in cells of endocervical type; cellular stratification but only when combined with nuclear changes; loss of nuclear polarity; nuclear enlargement, pleomorphism, hyperchromasia; mitotic activity, some of which may be abnormal forms; prominent nucleoli; apoptosis. It can usually be distinguished from microinvasive adenocarcinoma by its limitation to the glandular field, admixture of normal and abnormal glands, lack of stromal response and lack of cytological changes seen in microinvasive adenocarcinoma (increased pleomorphism, paler, more copious and eosinophilic cytoplasm). Invasion should not be excluded on small punch biopsies.
4In American terminology most often the term LEEP (Lus Electrosurgical Procedure) is used, whereas in the English literature, usually the term LLETZ (Large Loop Excision of the Transformation Zone) is used. In this guideline only LLETZ is used.
References
- Arbyn M, Herbert A, Schenck U, . European guidelines for quality assurance in cervical cancer screening: Recommendations for collecting samples for conventional and liquid-based cytology. Cytopathology 2007;18:133–9.
- Herbert A, Bergeron C, Wiener H, Schenck U, Klinkhamer PJ, Arbyn M. European guidelines for quality assurance in cervical cancer screening: Recommendations for cervical cytology terminology. Cytopathology 2007;18:213–9.
- Giordano L, Webster P, Szarewski A, . Improving the quality of communication in organised cervical cancer screening. Patient Educ Couns 2008;72:130–6.
- Arbyn M, Dillner J. Review of current knowledge on HPV vaccination: An appendix to the European guidelines for quality assurance in cervical cancer screening. J Clin Virol 2007;38:189–97.
- Jordan J, Arbyn M, Martin-Hirsch P, . European guidelines for quality assurance in cervical cancer screening: Recommendations for clinical management of abnormal cervical cytology, part 1. Cytopathology 2008;19:342–54.
- Jordan J, Martin-Hirsch P, Arbyn M, . European guidelines for management of abnormal cervical cytology, Part 2. Cytopathology 2009;20:5–16.
- Arbyn M, Anttila A, Jordan J, . European guidelines for quality assurance in cervical cancer screening. Second Edition – Summary document. Ann Oncol 2010;21:448–58.
- Costa MJ, Grimes C, Tackett E, Naib ZM. Cervicovaginal cytology in an indigent population. Comparison of results for 1964, 1981 and 1989. Acta Cytol 1991;35:51–6.
- Singer A, Monaghan JM. Lower genital tract precancer: Colposcopy, pathology and treatment. 2nd. Oxford: Blackwell Science Ltd.; 2000.
- European Commission. European guidelines for quality assurance in cervical cancer screening. 2nd. Luxembourg: Office for Official Publications of the European Communities; 2008.
- Jordan J, Martin-Hirsch P, Arbyn M, . Chapter 6: Management of abnormal cervical cytology. Arbyn M, Anttila A, Jordan J, Ronco G, Schenck U, Segnan N, ., European guidelines for quality assurance in cervical cancer screening. 2nd. Luxembourg, Office for Official Publications of the European Communities; 2008. 191–232.
- Obermair A, Wanner C, Bilgi S, . The influence of vascular space involvement on the prognosis of patients with stage IB cervical carcinoma: Correlation of results from hematoxylin and eosin staining with results from immunostaining for factor VIII-related antigen. Cancer 1998;82:689–96.
- Birner P, Obermair A, Schindl M, Kowalski H, Breitenecker G, Oberhuber G. Selective immunohistochemical staining of blood and lymphatic vessels reveals independent prognostic influence of blood and lymphatic vessel invasion in early-stage cervical cancer. Clin Cancer Res 2001;7:93–7.
- IARC. Cervix cancer screening. IARC handbooks of cancer prevention. Vol. 10. Lyon: IARC Press; 2005.
- WHO. Pathology and genetics of tumours of the breast and female genital organs. Lyon: IARC Press; 2003.
- Mitchell MF, Tortolero-Luna G, Wright TC, . Cervical human apillomavirus infection and intraepithelial neoplasia: A review. J Natl Cancer Inst Monogr 1996;17–25.
- Ostor AG, Mulvany N. The pathology of cervical neoplasia. Curr Opin Obstet Gynecol 1996;8:69–73.
- Melnikow J, Nuovo J, Willan AR, Chan BK, Howell LP. Natural history of cervical squamous intraepithelial lesions: A meta-analysis. Obstet Gynecol 1998;92:727–35.
- Herbert A, Arbyn M, Bergeron C. Why CIN3 and CIN2 should be distinguished on histological reports. Cytopathology 2008;19:63–4.
- Ismail SM, Colclough AB, Dinnen JS, . Observer variation in histopathological diagnosis and grading of cervical intraepithelial neoplasia. BMJ 1989;298:707–10.
- Stoler MH, Schiffman MA. Interobserver reproducibility of cervical cytologic and histologic interpretations. JAMA 2001;285:1500–5.
- Crum CP, Egawa K, Fu YS, . Atypical immature metaplasia (AIM). A subset of human papilloma virus infection of the cervix. Cancer 1983;51:2214–9.
- Klaes R, Benner A, Friedrich T, . p16INK4A immunohistochemistry improves interobserver agreement in the diagnosis of cervical intraepithelial neoplasia. Am J Surg Pathol 2002;26:1389–99.
- NHSCSP. Histopathology reporting in cervical screening. Working party of the Royal College of Pahtologists and the NHS Cervical Screening Programme. Shefield: NHS Cancer Screening Programmes; 1999. Report No.: 10.
- Goldstein NS, Ahmad E, Hussain M, Hankin RC, Perez-Reyes N. Endocervical glandular atypia: Does a preneoplastic lesion of adenocarcinoma in situ exist? Am J Clin Pathol 1998;110:200–9.
- Horn LC, Riethdorf L, Loning T. Leitfaden für die Präparation uteriner Operationspräprate. Pathologe 1999;20: 9–14.
- Robboy SJ, Kraus FT, Kurman RJ. Gross discription: Processing and reporting of gynecologic and obstretric specimens. Kurman RJ, Blaustein's pathology of the female genital tract. 4th. Springer-Verlag; 1994. 1225–40.
- Robboy SJ, Russell P, Anderson MC, Bentley RC. Cutup – the gross description, processsing and reporting of specimens. Pathol Female Reproduct Tract 2002;861–77.
- Heatley MK. How many histological levels should be examined from tissue blocks originating in cone biopsy and large loop excision of the transformation zone specimens of cervix? J Clin Pathol 2001;54:650–1.
- Breitenecker G, Wiener H, Stani J. Cervical cancer screening in Austria. Eur J Cancer 2000;36:2189–90.
- Kobak WH, Roman LD, Felix JC, Muderspach LI, Schlaerth JB, Morrow CP. The role of endocervical curettage at cervical conization for high-grade dysplasia. Obstet Gynecol 1995;85:197–201.
- Hoffman MS, Sterghos S Jr, Gordy LW, Gunasekar D. Evaluation of the cervical canal with the endocervical brush. Obstet Gynecol 1993;82:573–577.
- Mogensen ST, Bak M, Dueholm M, . Cytobrush and endocervical curettage in the diagnosis of dysplasia and malignancy of the uterine cervix. Acta Obstet Gynecol Scand 1997;76:69–73.
- Boardman LA, Meinz H, Steinhoff MM, Heber WW, Blume J. A randomized trial of the sleeved cytobrush and the endocervical curette. Obstet Gynecol 2003;101:426–30.
- Pretorius RG, Zhang WH, Belinson JL, . Colposcopically directed biopsy, random cervical biopsy, and endocervical curettage in the diagnosis of cervical intraepithelial neoplasia II or worse. Am J Obstet Gynecol 2004;191:430–4.
- Bulten J, van der Laak JA, Gemmink JH, Pahlplatz MM, de Wilde PC, Hanselaar AG. MIB1, a promising marker for the classification of cervical intraepithelial neoplasia. J Pathol 1996;178:268–73.
- Bulten J, de Wilde PC, Schijf C, . Decreased expression of Ki-67 in atrophic cervical epithelium of post-menopausal women. J Pathol 2000;190:545–53.
- Smela M, Chosia M, Domagala W. Proliferation cell nuclear antigen (PCNA) expression in cervical intraepithelial neoplasia (CIN). An immunohistochemical study. Pol J Pathol 1996;47:171–4.
- Klaes R, Friedrich T, Spitkovsky D, . Overexpression of p16(INK4A) as a specific marker for dysplastic and neoplastic epithelial cells of the cervix uteri. Int J Cancer 2001;92: 276–84.
- Anttila A, Ronco G, Lynge E, . Chapter 2: Epidemiological guidelines for quality assurance in cervical cancer screening. Arbyn M, Anttila A, Jordan J, Ronco G, Schenck U, Segnan N, . European guidelines for quality assurance in cervical cancer screening. 2nd Luxembourg, Office for Official Publications of the European Communities; 2008. 11–52.
- Ronco G, Anttila A. Chapter 7: Summary table of key performance indicators. Arbyn M, Anttila A, Jordan J, Ronco G, Schenck U, Segnan N, . European guidelines for quality assurance in cervical cancer screening, 2nd. Luxembourg, Office for Official Publications of the European Communities; 2008. 231–42.
- Vutuc C, Haidinger G, Waldhoer T, Ahmad F, Breitenecker G. Prevalence of self-reported cervical cancer screening and impact on cervical cancer mortality in Austria. Wien Klin Wochenschr 1999;111:354–9.
- Wiener HG, Klinkhamer P, Schenck U, . European guidelines for quality assurance in cervical cancer screening: Recommendations for cytology laboratories. Cytopathology 2007;18:67–78.
- FIGO. FIGO annual report on the results of treatment in gynaecological cancer. 24th. J Epidemiol Biostat 2000;Special issue:x.