Abstract
Background. Patients undergoing surgery for non-small cell lung cancer (NSCLC) are often elderly with co-morbid conditions and decreased performance status. Thus, the morbidity of lung resection via thoracotomy may be unacceptable for some patients. This is the reason why video-assisted thoracoscopic surgery (VATS) instead of open thoracotomy has gained more use and acceptance, especially in patients with stage I disease. The aim of this study was to evaluate the difference between VATS and open thoracotomy in treatment outcomes of stage I NSCLC patients. Methods. A total of 328 stage I NSCLC patients underwent lobectomy, bilobectomy or segmentectomy between January 2000 and February 2010. VATS was implemented in 116 patients, of which 16 were converted to thoracotomy. Muscle-sparing anterolateral thoracotomy was performed in 212. Propensity-matched groups were analyzed based on preoperative variables and stage. Results. VATS was associated with lower postoperative morbidity in both overall (p = 0.020) and propensity-matched analysis (p = 0.026) and shorter hospitalization (both p < 0.001). Patients selected for VATS were older (p = 0.001) with a significantly higher Charlson comorbidity index (p = 0.007) and poorer diffusion capacity (p < 0.001). The conversion rate was 14%. Between the two groups, no significant difference was observable in two-year overall and progression-free survival. Conclusions. Despite the VATS lobectomy and segmentectomy patients’ being older, with more comorbid condition and poorer pulmonary function, the incidence of major complications was lower and hospitalization shorter than for open thoracotomy patients. For stage I NSCLC, VATS should be considered the primary surgical approach.
Radical surgery offers the best chance for curative treatment in early stage non-small cell lung cancer (NSCLC). In clinical stage I disease, video-assisted thoracosopic surgery (VATS) lobectomy has been shown to reduce morbidity and offer equivalent oncologic outcome equivalent to those from open lung resection [Citation1]. Both open and VATS segmentectomy for T1a tumors have compared favorably with lobectomy, as well [Citation2,Citation3]. Despite this evidence, only 20% of pulmonary resections are currently done using either minimally invasive lobectomy or segmentectomy techniques. The main concerns have been impact on patient safety, feasibility of an en-bloc resection of the target lobe, and the possibility of locoregional tumor dissemination [Citation1].
Only few reports address whether the transition from open to VATS lobectomy can be performed safely [Citation4]. Published results of VATS lobectomy have originated mainly from North American or Asian institutions. Only a few European institutional reports exist [Citation5–7].
Implementation of VATS began in our unit in 1992 for staging and for benign diseases (J.S.), and for anatomic radical NSCLC resections, VATS began in March 2006. This study retrospectively reviews the outcomes of VATS lobectomy and segmentectomy compared to open lung resection in stage I NSCLC and evaluates the impact of increased use of VATS upon patient selection, hospitalization, and patient outcomes.
Patients and methods
Patients
Between January 2000 and February 2010, 622 patients underwent surgical treatment for lung cancer at the Division of General Thoracic and Esophageal Surgery in the Department of Cardiothoracic Surgery, Helsinki University Central Hospital. Of these 622 patients, 328 (52.7%) with clinical stage I NSCLC who underwent lobectomy, bi-lobectomy, or segmentectomy were included to this retrospective observational study. A total of 27 Stage I NSCLC patients were excluded because their lung resection included; either pneumonectomy, sleeve resection, or wedge resection. The institutional review board approved the study. Patient characteristics and clinical data were collected from individual patient records and then analyzed in an electronic database anonymously. A total of eight surgeons performed the operations, and two of them conducted VATS operations (E.S. and J.R.). Patients operated on with other operative techniques (pneumonectomy, sleeve resection, and wedge resection) were excluded. None of the patients evaluated received preoperative chemo- or radiotherapy. Patient characteristics are shown in .
Table I. Lung resection patient characteristics.
Every patient was preoperatively evaluated with pulmonary function tests and computed tomography (CT) of the chest and upper abdomen. Additional need for positron emission tomography (PET), bronchoscopy, mediastinoscopy, CT-guided fine-needle aspiration (FNA) biopsy, ventilation-perfusion scanning, exercise testing, and a stair-climbing test was decided on an individual basis. If no prior preoperative pathologic diagnosis was established, intraoperative frozen section specimens confirmed the pathologic diagnosis.
The status of comorbidity was objectively quantified based on the Charlson comorbidity index (CCI), which takes into account both the number and seriousness of the comorbid diseases and has been validated in lung cancer patients [Citation7] and previously used in VATS-operated NSCLC patients [Citation8]. Weighing of co-morbid conditions is presented in Charlson's article [Citation9].
Overall and progression-free survival at two years were assessed collectively in April 2010.
Surgical techniques
With the patient in the lateral decubitus position under single-lung ventilation, the 10-mm camera port is placed at the seventh or eighth intercostal space in the anterior axillary line. The second 10-mm port is placed four finger-breadths distance from the first port at the posterior axillary line (the seventh or eighth intercostal space). The utility incision (no larger than 4 cm in length) is placed directly over the superior pulmonary vein for upper lobectomies (approximately the third or fourth interspace), and one interspace lower for middle and lower lobectomies. An additional 5-mm port is placed right under the tip of the scapula if needed. No rib spreading is ever used. Open thoracotomies were conducted via anterolateral muscle-sparing thoracotomies without routine sectioning of a rib.
In VATS, the mediastinal lymph node dissection on the right side includes levels 4R, 7, and 9R. For left-sided resections, we routinely dissect levels 5, 6, 7, and 9L. Total mediastinal lymph node dissection was performed in open operations.
Statistical analysis
Propensity scores were estimated by a logistic model including the following variables: age, gender, Charlson co-morbidity index score, forced expiratory volume in one second of predicted, preoperative staging, and pack years smoked. Patients were matched between groups according to their propensity score. Patients converted from VATS to thoracotomy were grouped and analyzed in the VATS group and separately. For comparison of categorical variables, Fischer's exact or χ2 tests were used. For categorical variables and the 2-sample rank sum test, Mann-Whitney U served for continuous variables. Comparisons of survival were made by the log rank test. All tests for significance were two-sided, with p < 0.050 considered statistically significant. Data analysis was performed with PASW 18.0.1 (SPSS Inc, Chicago, IL, USA).
Results
A total of 328 patients were eligible for this study, of whom 212 underwent open thoracotomy and 116 underwent VATS. Operative and perioperative outcomes are presented in . No intraoperative deaths occurred. Two completed VATS patients (2%) were observed overnight in the intensive care unit, whereas 21 thoracotomy patients (9%) were treated in the intensive care unit for median five days (range: 1–36 days). There occurred six in-hospital deaths in the thoracotomy group (3%): three interstitial pneumonias, two cerebral infarctions, and one pneumonia. Also, there occurred three in-hospital deaths in VATS group, of two were intraoperatively converted to thoracotomy due to bleeding and adhesions. Both converted patients died following an intensive care period of 26 days for interstitial pneumonia, and 14 days for acute heart failure; and one non-converted VATS patient died at rehabilitation facility due to pulmonary embolus (). Conversion from VATS to thoracotomy was done in a total of 16 cases (14%). Indications for conversions were visualization (6), dense adhesions (5), bleeding (4), and sudden hypotension (1). No significant differences emerged in preoperative variables between converted and non-converted patients. Patient characteristics and results, divided into three patient groups with two-year intervals between 2004 and 2009, are presented in . No differences in overall survival or progression-free survival were observed at two years, between the VATS and the thoracotomy groups. Kaplan-Meier survival analysis comparing thoracotomy and VATS is presented in and divided by pathologic mediastinal status in .
Figure 1A-C. Kaplan-Meier analysis: Thoracotomy versus video-assisted thoracoscopic surgery, (A) All patients, (B) Node-negative patients and (C) Node-positive patients.
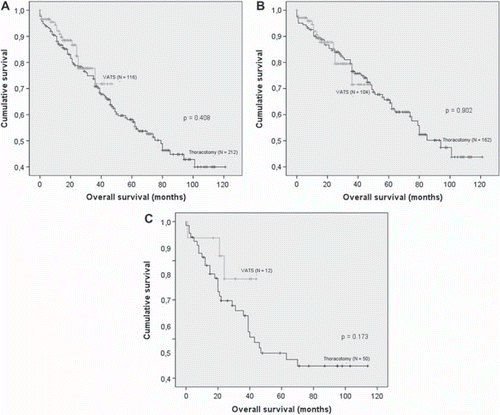
Table II. Operative and perioperative outcomes.
Table III. Postoperative complications, n (%).
Table IV. Comparison between patient characteristics and results, divided into two-year patient groups.
Discussion
According to our retrospective analysis of patients with clinical stage-I NSCLC, outcomes of VATS lobectomy and segmentectomy are similar to those of open surgery. The minimally invasive approach was, however, associated with fewer complications and a shorter postoperative hospital stay when assessed both directly and in a propensity score-based matched comparison. Furthermore, the implementation of these VATS techniques in a medium-sized thoracic unit was safe, with a decreased need for hospital beds and with an increased likelihood of providing surgical treatment for higher-risk patients.
In our study, those patients undergoing VATS were at higher surgical risk: They were older and had a higher CCI score or poorer DLCO. These findings reveal that VATS in our unit has been first implemented for patients having poorer overall health and being at higher risk. In practice, this may enable NSCLC patients who are deemed medically inoperable for open surgery based on age, pulmonary function, co-morbid conditions, or other factors to be suitable candidates for VATS lobectomy or segmentectomy. After VATS, patients face less pain [Citation10] and less lung function loss [Citation11]. With better lung function and less pain the risk of postoperative morbidity decreases, especially in high-risk patients. They recover faster with less need for additional support [Citation12]. We have shown that lobectomy and bi-lobectomy via thoracotomy is associated with a long-term decrease in postoperative quality of life [Citation13]. This long-term quality of life is, however, better after VATS lobectomy than after open surgery [Citation14].
In our 24-month and overall survival analysis, no significant difference appeared between patient groups, as seen similarly in other studies [Citation5,Citation6,Citation15]. The decreased postoperative hospitalization is well established with VATS [Citation16] and also now verified here. This is the main reason for the cost benefit from VATS, compared to open thoracotomy, because increased theater costs (disposables and time) are counterbalanced by a shorter hospital stay [Citation17]; the mean overall cost for VATS lobectomy in this study was 8023 Euros and for open thoracotomy 8178 Euros. These costs, however, exclude cost savings after discharge such as earlier return to work and less need for further support [Citation18].
The therapeutic effect of mediastinal dissection over sampling is highly debated. Of importance is the complete staging enabling better delivery of postoperative adjuvant therapy [Citation19]. In our series, we sampled more nodal stations in open surgery than in VATS. In the VATS group, more patients were, however, ineligible for adjuvant chemotherapy, either because of age or co-morbidity, and selective sampling was thus the choice. With experience, these techniques can be equivalent. Thus, mediastinal staging should be done routinely in patients eligible for adjuvant therapy despite the use of preoperative PET-CT.
The overall rate of complications related to minimally invasive surgery was lower than that of open surgery. It seems that VATS may cause less postoperative air leak than does open thoracotomy, probably due to greater use of staplers. Our study population in the VATS patient group comprised, however, only 116 patients, meaning that only a few complications were observable per complication category. This study is thus underpowered to determine differences in complication types between VATS and thoracotomy. This issue has been addressed recently by Paul et al. [Citation20] and Villamizar et al. [Citation12]. Overall, VATS has been a safe technique during the time it has been implemented in a medium-sized thoracic unit.
Our conversion rate of 14% from VATS to open thoracotomy was similar to that of other reports [Citation4,Citation21,Citation22]. Much lower rates have been reported [Citation1]. The most common indication for conversion in our series was the inability to localize small tumors for frozen section and nodal disease detected during VATS. We feel that in biopsy-confirmed nodal disease, especially in low-risk patients, a complete open nodal dissection is necessary. To lower the conversion rate, patients with small tumors, especially ones more centrally located, should undergo preoperative fine needle aspiration biopsy. Two converted patients died postoperatively. In our view this justifies for further studies in patients with poor performance status undergoing VATS. Thus, if there exists in high-risk VATS patients significant risk for conversion such as previous thoracic surgery or lymphadenopathy related to underlying interstitial lung disease, the operative morbidity of an open operation should also be weighed.
To deal with inherited biases of nonrandomized comparison, we analyzed the data by use of propensity score matching, which is a multivariate statistical method that identifies groups of patients with similar chances of receiving one treatment or another from within a given study population [Citation23]. It is not, however, equivalent to a prospective, randomized study comparing two surgical techniques with inherent bias.
In order to weigh the preoperative comorbid conditions for propensity matching, we implemented CCI, shown to be a better predictor of postoperative survival than are any individual comorbid conditions in patients undergoing surgery for NSCLC [Citation7]. It has been evaluated also in NSCLC patients undergoing surgery with a CCI score of two or more [Citation8] and together with propensity score analysis [Citation24]. The contribution of postoperative pain is neglected, as there exist no means for its reliable retrospective evaluation. Here, we have not taken into account surgeon-related factors. A possible selection bias is possible, because all VATS operations were performed by one of two surgeons, whereas six surgeons, including surgeons in training, performed only the conventional thoracotomy.
Despite recent developments in NSCLC treatment, it is still associated with poor overall survival in Finland. The recent study by Hakulinen et al. [Citation25], highlights this important issue and discusses the importance of early detection and treatment. In response to these demands, use of VATS in stage I NSCLC rose to 63%, during the four-year period, being similar to figures of Seder et al. [Citation4]; The rate of segmentectomies increased to 17%. With experience, the figures for minimally invasive surgery in the treatment of clinical stage I NSCLC can be over 90% [Citation6]. This optimizes the use of hospital beds and enables resection for a greater spectrum of NSCLC patients. Though with the implementation of VATS no significant change was apparent in CCI score and in patient age in the overall patient population, a decrease was apparent in preoperative pulmonary function tests. In addition to patients with poor lung function, VATS is nowadays a recommended approach for many other patient groups [Citation26].
Acknowledgements
The authors thank Mrs Yvonne Sundström for her excellent secretarial assistance. The authors declare no conflict of interest.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Yan TD, Black D, Bannon PG, McCaughan BC. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol 2009;27:2553–62.
- Shapiro M, Weiser TS, Wisnivesky JP, Chin C, Arustamyan M, Swanson SJ. Thoracoscopic segmentectomy compares favorably with thoracoscopic lobectomy for patients with small stage I lung cancer. J Thorac Cardiovasc Surg 2009;137:1388–93.
- Watanabe A, Ohori S, Nakashima S, Mawatari T, Inoue N, Kurimoto Y, . Feasibility of video-assisted thoracoscopic surgery segmentectomy for selected peripheral lung carcinomas. Eur J Cardiothorac Surg 2009;35:775–80; Discussion 780.
- Seder CW, Hanna K, Lucia V, Boura J, Kim SW, Welsh RJ, . The safe transition from open to thoracoscopic lobectomy: A 5-year experience. Ann Thorac Surg 2009;88: 216–25; Discussion 225–6.
- Roviaro G, Varoli F, Vergani C, Nucca O, Maciocco M, Grignani F. Long-term survival after videothoracoscopic lobectomy for stage I lung cancer. Chest 2004;126:725–32.
- Walker WS, Codispoti M, Soon SY, Stamenkovic S, Carnochan F, Pugh G. Long-term outcomes following VATS lobectomy for non-small cell bronchogenic carcinoma. Eur J Cardiothorac Surg 2003;23:397–402.
- Birim O, Maat AP, Kappetein AP, van Meerbeeck JP, Damhuis RA, Bogers AJ. Validation of the charlson comorbidity index in patients with operated primary non-small cell lung cancer. Eur J Cardiothorac Surg 2003;23:30–4.
- Nakanishi R, Yamashita T, Oka S. Video-assisted thoracic surgery lobectomy for non-small cell lung cancer in patients with a charlson comorbidity index score of two or more. J Thorac Oncol 2010;5:56–61.
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 1987; 40:373–83.
- Nagahiro I, Andou A, Aoe M, Sano Y, Date H, Shimizu N. Pulmonary function, postoperative pain, and serum cytokine level after lobectomy: A comparison of VATS and conventional procedure. Ann Thorac Surg 2001;72:362–5.
- Endoh H, Tanaka S, Yajima T, Ito T, Tajima K, Mogi A, . Pulmonary function after pulmonary resection by posterior thoracotomy, anterior thoracotomy or video-assisted surgery. Eur J Cardiothorac Surg 2010;37:1209–14.
- Villamizar NR, Darrabie MD, Burfeind WR, Petersen RP, Onaitis MW, Toloza E, . Thoracoscopic lobectomy is associated with lower morbidity compared with thoracotomy. J Thorac Cardiovasc Surg 2009;138:419–25.
- Ilonen IK, Räsänen JV, Knuuttila A, Sihvo EI, Sintonen H, Sovijärvi AR, . Quality of life following lobectomy or bilobectomy for non-small cell lung cancer, a two-year prospective follow-up study. Lung Cancer 2010;70:347–51.
- Aoki T, Tsuchida M, Hashimoto T, Saito M, Koike T, Hayashi J. Quality of life after lung cancer surgery: Video-assisted thoracic surgery versus thoracotomy. Heart Lung Circ 2007;16:285–9.
- Yamamoto K, Ohsumi A, Kojima F, Imanishi N, Matsuoka K, Ueda M, . Long-term survival after video-assisted thoracic surgery lobectomy for primary lung cancer. Ann Thorac Surg 2010;89:353–9.
- Swanson SJ, Herndon JE 2nd, D’Amico TA, Demmy TL, McKenna RJ Jr, Green MR, . Video-assisted thoracic surgery lobectomy: Report of CALGB 39802 – a prospective, multi-institution feasibility study. J Clin Oncol 2007;25: 4993–7.
- Casali G, Walker WS. Video-assisted thoracic surgery lobectomy: Can we afford it? Eur J Cardiothorac Surg 2009;35: 423–8.
- Demmy TL, Plante AJ, Nwogu CE, Takita H, Anderson TM. Discharge independence with minimally invasive lobectomy. Am J Surg 2004;188:698–702.
- Petersen RP, Pham D, Burfeind WR, Hanish SI, Toloza EM, Harpole DH Jr, . Thoracoscopic lobectomy facilitates the delivery of chemotherapy after resection for lung cancer. Ann Thorac Surg 2007;83:1245–9; Discussion 1250.
- Paul S, Altorki NK, Sheng S, Lee PC, Harpole DH, Onaitis MW, . Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: A propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010;139:366–78.
- Handy JR Jr, Asaph JW, Douville EC, Ott GY, Grunkemeier GL, Wu Y. Does video-assisted thoracoscopic lobectomy for lung cancer provide improved functional outcomes compared with open lobectomy? Eur J Cardiothorac Surg 2010;37:451–5.
- Flores RM, Park BJ, Dycoco J, Aronova A, Hirth Y, Rizk NP, . Lobectomy by video-assisted thoracic surgery (VATS) versus thoracotomy for lung cancer. J Thorac Cardiovasc Surg 2009;138:11–8.
- Blackstone EH. Comparing apples and oranges. J Thorac Cardiovasc Surg 2002;123:8–15.
- Scott WJ, Allen MS, Darling G, Meyers B, Decker PA, Putnam JB, . Video-assisted thoracic surgery versus open lobectomy for lung cancer: A secondary analysis of data from the American college of surgeons oncology group Z0030 randomized clinical trial. J Thorac Cardiovasc Surg 2010; 139:976–81; Discussion 981–3.
- Hakulinen T, Engholm G, Gislum M, Storm HH, Klint A, Tryggvadóttir L, Bray F. Trends in the survival of patients diagnosed with cancers in the respiratory system in the Nordic countries 1964–2003 followed up to the end of 2006. Acta Oncol 2010;49:608–23.
- Demmy TL, Nwogu C. Is video-assisted thoracic surgery lobectomy better? Quality of life considerations. Ann Thorac Surg 2008;85:S719–28.
