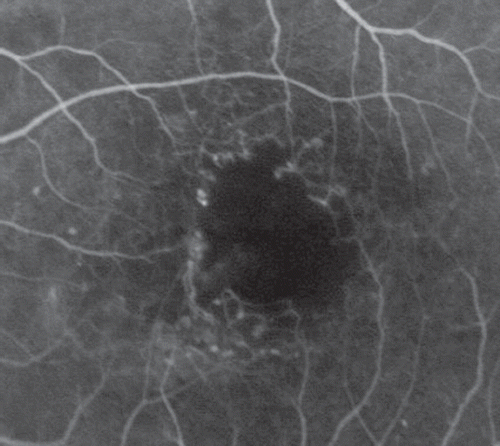
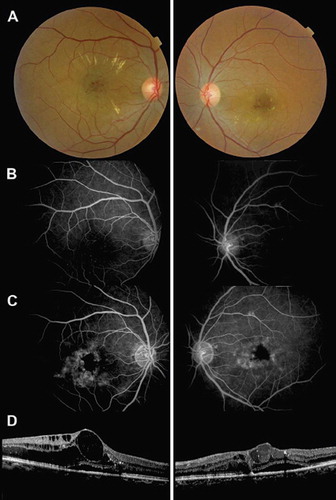
Trastuzumab (Herceptin, Genentech Inc) is a monoclonal antibody interfering with human epidermal growth factor receptor 2, which is currently used for the treatment of breast cancer [Citation1] and metastatic gastric cancer [Citation2]. We report a case of bilateral ischemic maculopathy following intravenous therapy of trastuzumab in a 50-year-old woman. She had breast cancer with cerebral and pulmonary metastases and had been treated with combined cerebral radiotherapy and docetaxel therapy without visual side effects, as confirmed by a normal ophthalmologic examination performed at the time. Trastuzumab (2 mg/kg) was introduced six months after this first-line radiochemotherapy regimen. Three months later, she presented with severe bilateral visual loss: visual acuity (VA) was 20/100 and 20/200 (right eye and left eye, respectively). On funduscopy, macular edema, hemorrhages, and hard exudates were noted in both eyes (), while the retinae beyond the vascular arcades were normal. On fluorescein angiography (FA), the foveal avascular zone was enlarged in both eyes (). Leakage from the unperfused foveolar capillaries and cystoid macular edema were also present (). Optical coherence tomography (OCT) examination showed bilateral cystic changes and shallow central serous retinal detachment (). Central macular thickness (CMT) was measured at 546 μm and 332 μm in the right and left eyes, respectively. VA worsened and CMT increased under trastuzumab treatment. As a consequence, trastuzumab was discontinued for three months. No progression was observed. One month after its reintroduction, vision decreased again (20/300, 20/400), new areas of capillary dropout appeared (), and CMT increased (629 μm, 450 μm).
Figure 1 A. Color fundus images 1 month after the initiation of trastuzumab: small hemorrhages and hard exudates were present in the macular area of both eyes. B. Early phase of the fluorescein angiography (FA) showing a significant enlargement of the foveal avascular zone. C. The late-phase of FA showed dye leakage from the perifoveal capillaries. D. Spectral-domain OCT showed bilateral cystic macular edema associated with serous retinal detachment.
Comment
Radiotherapy and chemotherapy can both induce ischemic maculopathy [Citation3]. A 45-Gy dose of radiation results in only 5% retinal complications [Citation4]. In this case, the patient underwent fractionated (10 fractions) low-dose radiotherapy (25 Gy). Only one-third of the eyeball was irradiated. Moreover, retinal periphery was normal and there was no radiation cataract. Therefore, radiotherapy is unlikely to be responsible for the ischemia. Docetaxel toxicity can also be ruled out: docetaxel-induced macular edema is typically not associated with capillary leakage and is reversible after treatment interruption [Citation5].
The onset of maculopathy after treatment initiation and its worsening after its resumption may suggest that trastuzumab is involved. Trastuzumab is a potent antiangiogenic agent reducing pro-angiogenic factor expression such as vascular endothelial growth factor, transforming growth factor-α, and plasminogen activator inhibitor-1 [Citation6]. Cases of ischemic maculopathy have been reported after anti-VEGF intravitreal injections [Citation7], and trastuzumab is known to induce capillary caliber reduction [Citation6] and leakage [Citation8]. Macular ischemia has not been reported as a side effect of trastuzumab. However, retinal and/or visual disorders were noted in 136 (1.93%) of the 7042 reports from the FDA and the community for Herceptin [Citation9]. Some of these reported disorders might be related to macular ischemia.
Conclusion
Macular ischemia often leads to legal blindness. As no effective treatment is available for this condition, ophthalmologic follow-up should be recommended for patients treated with trastuzumab, and drug discontinuation should be considered in case of ischemic maculopathy.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Guarneri V, Barbieri E, Dieci MV, Piacentini F, Conte P. Anti-HER2 neoadjuvant and adjuvant therapies in HER2 positive breast cancer. Cancer Treat Rev 2010;36 (Suppl 3):S62–6.
- De Vita F, Giuliani F, Silvestris N, Catalano G, Ciardiello F, Orditura M. Human epidermal growth factor receptor 2 (HER2) in gastric cancer: A new therapeutic target. Cancer Treat Rev 2010;36(Suppl 3):S11–5.
- Parsons JT, Bova FJ, Fitzgerald CR, Mendenhall WM, Million RR. Radiation retinopathy after external-beam irradiation: Analysis of time-dose factors. Int J Radiat Oncol Biol Phys 1994;30:765–73.
- Horgan N, Shields CL, Mashayekhi A, Shields JA. Classification and treatment of radiation maculopathy. Curr Opin Ophthalmol 2010;21:233–8.
- Telander DG, Sarraf D. Cystoid macular edema with docetaxel chemotherapy and the fluid retention syndrome. Semin Ophthalmol 2007;22:151–3.
- Izumi Y, Xu L, di Tomaso E, Fukumura D, Jain RK. Tumour biology: Herceptin acts as an anti-angiogenic cocktail. Nature 2002;416(6878):279–80.
- Sabet-Peyman EJ, Heussen FM, Thorne JE, Casparis H, Patel SJ, Do DV. Progression of macular ischemia following intravitreal bevacizumab. Ophthalmic Surg Lasers Imaging 2009;40:316–8.
- Witzel ID, Muller V, Harps E, Janicke F, Dewit M. Trastuzumab in pregnancy associated with poor fetal outcome. Ann Oncol 2008;19:191–2.
- eHealthme-real world drug outcomes (internet database). Herceptin reports. Available on http://www.ehealthme.com/.