Abstract
Aims/hypothesis. To further investigate the association of cancer occurrence with the use of insulin glargine. Methods. We followed 114 838 individuals using insulin between 1 July and 31 December 2005. From 1 January 2006 to 31 December 2008, we noted the occurrence of malignancies (cohort I). Insulin users between 1 July and 31 December 2006 were followed for the occurrence of malignancies in 2007 and 2008 (cohort II). Users of insulin during three consecutive six-month periods from 1 July 2005 to 31 December 2006 were followed for the occurrence of malignancies in 2007 and 2008 (cohort III). The Prescribed Drug Register, the Cancer Register, and the Causes of Death Register were used to obtain information on targeted person-time and outcome. We retrieved variables reflecting potential confounding factors from the Swedish National Diabetes Register, the Prescribed Drug Register, the Patient Register, the Medical Birth Register and the National Education Register. With Poisson regression we evaluated the association between insulin use and malignancy outcome with adjustment for confounders. Results. The adjusted incidence rate ratio (and 95% confidence interval) for women who used insulin glargine alone compared with those who used other types of insulin, was 1.60 (1.10–2.32) for breast cancer but included 1.0 for malignancy outcomes other than breast cancer for men and women when analyzing cohort I with follow-up in 2006–2008. For cohort II and III the corresponding incidence rate ratios were 1.38 (0.87–2.18), and 0.87 (0.41–1.85), respectively. Conclusion/interpretation. We do not see an increased risk during 2008 for breast cancer in the insulin glargine group. We need data for additional years before we can state with reasonable certainty that the increase in breast cancer incidence that we observed in Sweden in 2006 and 2007 was due to a random fluctuation or whether there is an association with the use of insulin glargine.
We have previously published, at the request of the European Association for the Study of Diabetes (EASD), cancer incidence data among insulin glargine users; during 2006 and 2007 we found an increased risk of breast cancer in Swedish women using insulin glargine alone [Citation1]. Investigators utilizing the Scottish diabetes clinical database also found a clear association between use of insulin glargine and breast cancer occurrence [Citation2]. No association was reported from a study based on data from UK General Practices [Citation3]. A German study found a decreased risk of all types of cancer among users of insulin glargine before adjusting for insulin dose, after adjustment the risk became increased [Citation4]. A study based on the insulin glargine manufacturer's pharmacovigilance databases for all randomized clinical trials did not find an association between insulin glargine and increased incidence of cancer, including breast cancer [Citation5]. A nested case-control study from Italy found that a possible association between cancer and higher glargine doses [Citation6]. We need more data to understand whether or not the reported associations between insulin glargine and cancer occurrence are causal or due to bias, reversed causality or happened by chance [Citation7–9].
We have now updated our previously reported cohort with cancer incidence data for 2008, with 50% more follow-up time. In addition we have defined two separate new, in part overlapping, cohorts to further investigate the association of breast cancer, gastrointestinal cancer, colorectal cancer, prostate cancer, pancreatic cancer and any type of malignancy with the use of insulin glargine.
Methods
We used the unique personal identity number assigned to each Swedish resident to link together information from seven population-based registers. The Prescribed Drug Register, the Cancer Register, and the Causes of Death Register were used to obtain information on targeted person-time and outcome. We retrieved variables reflecting potential confounding factors from the Swedish National Diabetes Register, the Prescribed Drug Register, the Patient Register, the Medical Birth Register and the National Education Register, for details see Jonasson et al. [Citation1] and .
The Swedish Prescribed Drug Register contains details of all the prescriptions dispensed in Sweden since 1 July 2005 [Citation10]. Patients with at least one filled prescription of insulin constituted our study cohort. The Swedish Cancer Register encloses since 1958 everyone who has been diagnosed with a new primary malignancy, including in situ tumors, and gave information on outcome. The completeness of the cancer register is very high [Citation11].
Ethical considerations
This study was approved by the regional ethics committee in Göteborg (DNR: 612-08).
Targeted person-time
We studied three separate cohorts. Cohort I is the same as in our previous publication, with an added year of follow-up [Citation1]. We studied 114 838 individuals who were 35–84 years old at the end of 2005, had at least one prescription dispensed for insulin (Anatomical Therapeutic Chemical (ATC) [Citation12] code A10A) between 1 July and 31 December 2005, and were alive at the start of follow-up (1 January 2006). We studied the first diagnosis of a primary malignancy as an outcome measure, excluding individuals who received this diagnosis at any time between 1 January 1958 and 31 December 2005. We followed the subjects from 1 January 2006 to 31 December 2008. Follow-up was from 1 January 2006 to death, loss to follow-up (censorship), the outcome being analyzed occurring, or end of study.
Cohort II, an identical approach to Cohort I but shifted one year, i.e. we studied 118 826 individuals who were 35–84 years old at the end of 2006, had at least one prescription dispensed for insulin between 1 July and 31 December 2006, and were alive at the start of follow-up (1 January 2007). We followed the subjects from 1 January 2007 to 31 December 2008 for diagnosis of a primary malignancy as in Cohort I.
In Cohort III we studied 102 883 individuals who were 35–84 years old at the end of 2005, had at least one prescription of insulin filled during three consecutive six-month periods from 1 July 2005 to 31 December 2006, and were alive at the start of follow-up (1 January 2007), i.e. continuous use of insulin for at least 18 months before start of follow-up. We followed the subjects from 1 January 2007 to 31 December 2008. We examined the first diagnosis of a primary malignancy in the exact same manner as in the analysis of Cohort I and II.
Categories of insulin use
Information on exposure to insulin and analogues for Cohort I was obtained from prescriptions dispensed between 1 July and 31 December 2005, for Cohort II between 1 July and 31 December 2006, and for Cohort III between 1 July 2005 and 31 December 2006. In Cohort III, individuals had to have at least one prescription of insulin during three consecutive six-month periods. Individuals entered as having prescriptions dispensed for insulin glargine (ATC code A10AE04) but no prescriptions dispensed for other types of insulin (ATC code A10A) were classified as using insulin glargine alone (no other types of insulin). Having prescriptions dispensed for both insulin glargine and another type of insulin classified the individual as a user of insulin glargine and other types of insulin. Having prescriptions dispensed for insulin but not insulin glargine classified the individual as a user of types of insulin other than insulin glargine.
Outcomes
Apart from “All malignant tumors” and “All malignant tumors and in situ tumors”, we studied: ‘breast cancer’ (International Classification of Diseases, 10th revision (ICD-10) code C50), ‘prostate cancer’ (C61), ‘gastrointestinal cancer’ (C16–C20), ‘colorectal cancer’ (C18-C20), ‘pancreatic cancer’ (C25). For these five sites only, we restricted the inclusion to tumors that were histopathologically classified as adenocarcinoma (WHO/HS/CANC/24.1 histology code 096).
Individuals entered with any type of malignancy between 1 January 1958 (the date the Cancer Register started) and 31 December 2005 (or 31 December 2006 in Cohort II and III) were excluded from the analysis when ‘any type of malignancy’ was the outcome. When studying a specific cancer, we excluded those who had previously been diagnosed with the specific cancers. We took the date of death from the Cause of Death register. As we did not have information on migration we classified subjects who were not entered as dead and who did not have a prescription dispensed for any drug in 2009 as having been lost to follow-up. The date for loss to follow-up was set at 90 days after the last date for a dispensed prescription, not exceeding end of study on 31 December 2008.
The number of subjects in the present analysis does not exactly match Jonasson et al, due to register updates correcting information or adding new late entries [Citation1].
Variables reflecting potential confounding factors
Sex and age were retrieved from the Prescribed Drug Register. We obtained data on age at onset of diabetes from the National Diabetes Register or estimated it from the time for first admission to hospital care with diabetes as main diagnosis (ICD-8 code 250; ICD-9 code 250; ICD-10 codes E10–E14) from data in the Patient Register for 1969 to 2005. An age at onset of diabetes of less than 30 years, as recorded in National Diabetes Register (primary choice) or by data from the Patient Register (secondary choice), defined an individual as having type 1 diabetes; an age at onset above 30 years defined an individual as having type 2 diabetes; the absence of information on age at onset defined the individual as having missing information on type of diabetes. Age at onset was used to calculate duration. For cohort I the highest BMI reported to the National Diabetes Register from 2003 to 2005 for each individual was used as the value for BMI. We retrieved information on smoking habits from 2003 to 2005 from the National Diabetes Register. Anyone who reported smoking during 2005 was classified as a current smoker. Anyone who reported not smoking in 2005 but reported smoking in 2003 or 2004 was classified as a former smoker. Anyone who reported not smoking in 2003, 2004 and 2005 was classified as a non-smoker. A record of a prescription dispensed for an oestrogen or for metformin in the Prescribed Drug Register from 1 July to 31 December 2005 defined oestrogen and metformin use, respectively.
A record of at least one hospital admission with a main diagnosis of any cardiovascular disease (ICD-10 codes I00–I99) in the National Patient Register, during the period 1 July 2004 to 30 June 2005 (i.e. one year prior to definition of exposure), classified the individual as having cardiovascular disease.
Educational level refers to the highest attained educational level at the end of 2005. Educational level was classified into the following three categories, representing distinct levels in the Swedish educational system: 1) 9 years or less of schooling, equivalent to elementary school or less; 2) 10–12 years of schooling, equivalent to secondary school; and 3) more than 12 years, equivalent to university. Age at birth of first child (women only) was categorized into no children, <30 years, ≥30 years, and missing information. A large group of women (49%), mainly the older women in the study population, had information missing on childbearing. For cohort II and cohort III the classification was the same but with data on covariates shifted one year to the right (e.g. 2004–2006 for smoking status).
Statistical methods
As a measure of the relative occurrence of malignancies, we used the incidence rate ratio (IRR). For example, we calculated the incidence rate of having been diagnosed with any type of malignancy among users of insulin glargine alone and compared it with the incidence rate among users of other types of insulin. We used Poisson regression analyses to evaluate the association between the three groups of insulin users and malignancy outcome. These models were fitted with the logarithm of observed person-time as the offset and they also provided 95% confidence intervals (CIs) of the incidence rate ratio. The Genmod procedure in the SAS statistical software package (SAS Institute, Cary, N.C., USA) was used for the calculations.
Results
shows the characteristics of the study subjects at baseline. Of the 114 838 subjects followed in Cohort I, 5 971 (5.2%) were classified as users of insulin glargine alone, 20 315 (17.7%) were classified as users of insulin glargine in combination with other types of insulin, and 88 552 (77.1%) were classified as users of types of insulin other than insulin glargine. The majority (91%) of users of insulin glargine alone were classified as having type 2 diabetes or as lacking information on the type of diabetes; a similar percentage (90%) was found for users of types of insulin other than insulin glargine. Users of insulin glargine in combination with other types of insulin had a lower mean age, and a lower percentage (61%) had type 2 diabetes or lacked information on the type of diabetes, than the other two groups. The distribution of the covariates in the exposure categories in Cohort II and Cohort III are similar to those in Cohort I.
Table I. Baseline characteristics for insulin users with at least one dispensed prescription between 1 July and 31 December 2005 (cohort I), between 1 July and 31 December 2006 (cohort II), and per six-month period between 1 July 2005 and 31 December 2006 (cohort III).
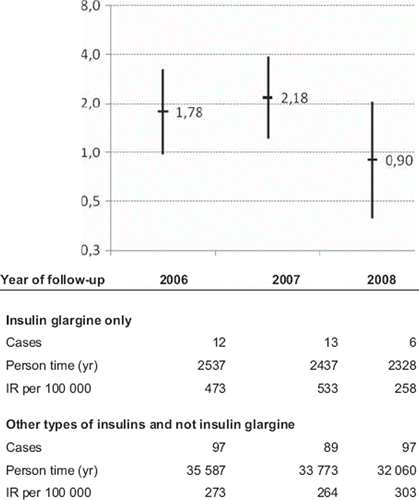
For malignancy outcomes other than breast cancer, number of cases, person-time and the 95% confidence intervals of the age-and-sex-adjusted incidence rate ratios for insulin glargine use were in the vicinity of 1.0 when analyzing follow-up in 2006–2008 (Appendix Table 1a and b: available in the online version of the journal. Please find this material with the direct link to the article: http://www.informahealthcare.com/doi/abs/10.3109/0284186X.2011.558913. The year-specific age-adjusted incidence rate ratios for breast cancer in women for insulin glargine use alone compared with insulin use other than insulin glargine for Cohort I are; 1.78 (0.97–3.24), 2.18 (1.21–3.90), and 0.90 (0.39–2.06) for the years 2006, 2007, and 2008 respectively ().
Figure 2. Incidence rate ratios (IRR) of breast cancer among users of insulin glargine only compared with users of other insulins and not insulin glargine. Yearly IRRs for the follow-up period 2006–2008. Users of insulin with at least one dispensed prescription between 1 July and 31 December 2005 (cohort I).
shows the sex and age-adjusted incidence rate ratios for those who used insulin glargine alone compared with those who used types of insulin other than insulin glargine. The first column shows cohort I with follow-up for 2006 and 2007, and in the second column we have added 2008 to the follow-up period, giving an incidence rate ratio of 1.60 (1.10–2.32). The third column shows the incidence rate ratios for Cohort II, mirroring the analyses in Cohort I in the first column but shifted one year, 1.38 (0.87–2.18). The last column represents Cohort III, for which exposure is defined during three consecutive six-month periods from 1 July 2005 to 31 December 2006, with follow-up until 2008, 0.87 (0.41–1.85).
Table II. Incidence rate ratio of cancer among users of insulin glargine alone compared with users of other types of insulins and not insulin glargine. Adjusted for age and sex.
All confidence intervals included 1.0 in the corresponding analyses for subjects who used insulin glargine in combination with other types of insulin compared with those who used types of insulin other than insulin glargine (Appendix Table 2: available in the online version of the journal. Please find this material with the direct link to the article: http://www.informahealthcare.com/doi/abs/10.3109/0284186X.2011.558913.
The age-adjusted incidence rate ratio for breast cancer presented in is adjusted for potential confounders, age, age at first child birth, age at onset of diabetes, body mass index, cardiovascular disease, metformin, oestrogen, smoking and educational level. As can be seen, additional adjustment made little or no change of the point estimates. Including duration or type of diabetes instead of age at onset did not change the point estimates. Analyzing users of insulin glargine only with a known age at onset after age 30, representing type 2, yielded an age-adjusted incidence rate ratio of 1.59 (1.00–2.52) and a multi-adjusted incidence rate ratio of 1.54 (0.97–2.44) (data not shown in table).
Table III. Incidence rate ratio of breast cancer among women using insulin glargine only, or insulin glargine and other insulins compared with women using other insulins and not insulin glargine. Insulin use 1 July to 31 December 2005, follow-up 2006–2008.
Discussion
With a follow-up in 2006, 2007 and 2008, the incidence rate ratio of breast cancer is lower than when we observed 2006 and 2007 only. In 2008 we noted an incidence of breast cancer among users of insulin glargine alone similar to that of users of other types of insulin and not insulin glargine. An analysis mirroring the analysis in our previous publication, defining exposure during 2006 and follow-up in 2007 and 2008, did not yield a statistically significant increased incidence of breast cancer for women who used insulin glargine alone compared with those who used types of insulin other than insulin glargine. A more sharply defined categorization of exposure, continuous use of insulin for at least 18 months before start of follow-up, produced an incidence rate ratio close to unity. This update adds to the impression that the increased incidence of breast cancer that we previously reported could be due to a random fluctuation in the studied Swedish population and did not reflect an actual effect of insulin glargine.
Several circumstances support the lack of an effect of insulin glargine on breast cancer incidence. The German study that started this discussion did not give us site-specific information [Citation4]. We do not know of any agent inducing malignancy in all organs, as the German group suggests insulin glargine does. The preclinical rationale for an effect (the closer resemblance between insulin glargine and insulin-growth factor 1 than between other types of insulin and insulin-growth-factor 1) can reasonably be applied to all organs for which there is an indication that insulin-growth factor 1 stimulates growth – probably including the prostate, colon, rectum and pancreas. We, as well as a Scottish author group, found incidences near the background level among insulin users for these other cancer sites [Citation1,Citation2]. Moreover, both studies also found incidences in the vicinity of the level among other insulin users for breast cancer when the subjects combined insulin glargine with other types of insulin – we know of no proposed mechanism for how an addition of other types of insulin would eliminate the hypothesized carcinogenic action of insulin glargine. Circulating levels of insulin-growth factor 1 are shown to be more than 100 times the levels of circulating insulin glargine, i.e. a possible extra contribution by insulin glargine to circulating insulin-growth factor 1 would probably be very small [Citation13–15]. Also, duration from the start of exposure of industrial agents to an excess risk of cancer is usually 10–25 years. We are not aware of any documentation of an increased incidence of cancer the year after initiation of treatment with a specific drug. Insulin glargine appeared on the Swedish market in 2003. The short duration from the start of insulin glargine use to the increased incidence rate for breast cancer suggests that our results could be due to random fluctuation.
We see no significant increase in incidence rate with increasing number of dispensed prescriptions of insulin glargine, speaking against a dose-response association [Citation1]. However, a nested case-control study from Italy found a possible association between cancer and higher glargine doses [Citation6]. Continuous use of insulin for at least 18 months before start of follow-up, i.e. a longer duration of exposure, produced an incidence rate ratio close to unity for the occurrence of breast cancer in 2007 and 2008. The collective randomized controlled trial experience of malignancies in studies using insulin glargine does not indicate an effect on cancer incidence by insulin glargine [Citation5].
However, at this point we cannot rule out the possibility that insulin glargine influences breast cancer growth. There is still a statistically significant increase in incidence when considering 2006, 2007 and 2008 together; the occurrence during 2008 may have been low due to a random fluctuation. The results concerning breast cancer were reproduced in Scotland [Citation2]. The biological rationale that insulin glargine, or other types of non-human insulin, may resemble insulin-growth factor 1 more than human insulin and the indication of a higher mitogenic potential of insulin glargine compared to human insulin in breast cancer cells deserves attention [Citation16–19]. In vitro, breast-cancer mitogenicity may be higher in serum from subjects taking insulin glargine than in serum from subjects taking other types of insulin [Citation20].
We performed extensive sensitivity analyses and found no indication that methodological issues distort our description of the relative incidences of breast cancer. A high percentage, in the vicinity of 85%, of women 40 to 74 years old regularly undergo mammography screening to diagnose small malignant tumors in the breast – the setting is working for early detection of agents stimulating growth of established tumors. The small changes in the incidence rate ratios that we obtained after adjustment indicate that our results probably cannot be explained by an imbalance in risk factors between users of insulin glargine and other types of insulin. Our population-based setting and fixed-cohort analysis avoid selection-induced problems. All cause mortality was lower among insulin glargine only users, as was acute myocardial infarction [Citation1], thus we have no clear indication of reversed causality.
We cannot account for factors causing breast cancer for which we have no knowledge or information. An unaccounted confounder needs to be strongly associated with both insulin glargine use and breast cancer, and not other types of cancer, and unrelated to the other studied covariates, to explain our results. We do not have any information on the use of insulin glargine or other types of insulin before 1 July 2005 and cannot further study intake over time. Insulin glargine was reimbursed in Sweden 1 June 2003. According to market research performed at the nationally owned pharmacies the split between Type 1 and Type 2 was 50/50 in end of 2007 and has remained similar until now. Hospital-based initiation will not be captured but once discharged the patient will have to have a prescription filled and will thus be captured in the register.
Our blunt classification of type 1 and type 2 diabetes certainly involves a large error. Observational studies show diabetes type 1 and 2 implicates varying cancer risks for varying sites; we do not know to what extent, if any, diabetes type modifies the effect when a drug induces cancer.
We have previously reported on an increased incidence of breast cancer in 2006 and 2007 in Sweden among users of insulin glargine, in this update we do not see an increased risk during 2008. Several critical examinations of the results concerning insulin glargine have been published [Citation7,Citation21–24]. We await with interest new results from other settings. We need data for 2009 and probably some additional years before we can state with reasonable certainty that the increase in breast-cancer incidence that we observed in Sweden in 2006 and 2007 happened by chance and not since insulin glargine increases breast-cancer risk.
Appendix Table 2
Download PDF (28.4 KB)Acknowledgements
The authors would like to thank Astrid and David Hagelén Foundation, Swedish Research Council, Swedish Diabetes Foundation, AGFOND, Konrad and Helfrid Johansson Foundation and VINNMER, VINNOVA for their support.
Declaration of interest: There are no conflicts of interest to be declared.
References
- Jonasson JM, Ljung R, Talbäck M, Haglund B, Gudbjörnsdottir S, Steineck G. Insulin glargine use and short-term incidence of malignancies – a population-based follow-up study in Sweden. Diabetologia 2009;52:1745–54.
- Colhoun HM. Use of insulin glargine and cancer incidence in Scotland: A study from the Scottish Diabetes Research Network Epidemiology Group. Diabetologia 2009;52:1755–65.
- Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia 2009;52:1766–77.
- Hemkens LG, Grouven U, Bender R, Gunster C, Gutschmidt S, Selke GW, . Risk of malignancies in patients with diabetes treated with human insulin or insulin analogues: A cohort study. Diabetologia 2009;52:1732–44.
- Home PD, Lagarenne P. Combined randomised controlled trial experience of malignancies in studies using insulin glargine. Diabetologia 2009;52:2499–506.
- Mannucci E, Monami M, Balzi D, Cresci B, Pala L, Melani C, . Doses of insulin and its analogues and cancer occurrence in insulin-treated type 2 diabetic patients. Diabetes Care 2010.
- Hernandez-Diaz S, Adami HO. Diabetes therapy and cancer risk: Causal effects and other plausible explanations. Diabetologia 2010;53:802–8.
- Renehan A, Smith U, Kirkman MS. Linking diabetes and cancer: A consensus on complexity. Lancet 2010;375:2201–2.
- Smith U, Gale EA. Cancer and diabetes: Are we ready for prime time? Diabetologia 2010;53:1541–4.
- Wettermark B, Hammar N, Ford CM, Leimanis A, Otterblad Olausson P, Bergman U, . The new Swedish Prescribed Drug Register – opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf 2007;16:726–35.
- Barlow L, Westergren K, Holmberg L, Talbäck M. The completeness of the Swedish Cancer Register: A sample survey for year 1998. Acta Oncol 2009;48:27–33.
- WHO Collaboration Centre for Drugs Statistics Methodology. Available from: http://www.whocc.no/atcddd/. Accessed 2010 Feb 10.
- Keay SD, Liversedge NH, Akande VA, Mathur RS, Jenkins JM. Serum IGF-1 concentrations following pituitary desensitization do not predict the ovarian response to gonadotrophin stimulation prior to IVF. Hum Reprod 2003;18:1797–801.
- Lepore M, Pampanelli S, Fanelli C, Porcellati F, Bartocci L, Di Vincenzo A, . Pharmacokinetics and pharmacodynamics of subcutaneous injection of long-acting human insulin analog glargine, NPH insulin, and ultralente human insulin and continuous subcutaneous infusion of insulin lispro. Diabetes 2000;49:2142–8.
- Strasburger CJ, Bidlingmaier M, Wu Z, Morrison KM. Normal values of insulin-like growth factor I and their clinical utility in adults. Horm Res 2001;55(Suppl 2):100–5.
- Mayer D, Shukla A, Enzmann H. Proliferative effects of insulin analogues on mammary epithelial cells. Arch Physiol Biochem 2008;114:38–44.
- Shukla A, Grisouard J, Ehemann V, Hermani A, Enzmann H, Mayer D. Analysis of signaling pathways related to cell proliferation stimulated by insulin analogs in human mammary epithelial cell lines. Endocr Relat Cancer 2009; 16:429–41.
- Weinstein D, Simon M, Yehezkel E, Laron Z, Werner H. Insulin analogues display IGF-I-like mitogenic and anti-apoptotic activities in cultured cancer cells. Diabetes Metab Res Rev 2009;25:41–9.
- Agin A, Jeandidier N, Gasser F, Grucker D, Sapin R. Glargine blood biotransformation: In vitro appraisal with human insulin immunoassay. Diabetes Metab 2007; 33:205–12.
- Mayer D, Chantelau E. Treatment with insulin glargine (Lantus) increases the proliferative potency of the serum of patients with type-1 diabetes: A pilot study on MCF-7 breast cancer cells. Arch Physiol Biochem 2010; 116:73–8.
- Butler PC. Insulin glargine controversy: A tribute to the editorial team at Diabetologia. Diabetes 2009;58: 2427–8.
- Gale EA. Insulin glargine and cancer: Another side to the story? Lancet 2009;374:521.
- Pocock SJ, Smeeth L. Insulin glargine and malignancy: An unwarranted alarm. Lancet 2009;374:511–3.
- Smith U, Gale EA. Does diabetes therapy influence the risk of cancer? Diabetologia 2009;52:1699–708.