Abstract
Background. Women with breast cancer (BC) report cognitive impairment. Receiving a BC diagnosis may have a negative psychological impact. We sought to determine whether a diagnosis of BC and subsequent surgical treatment reduced cognitive function. Material and methods. We recruited women, who had a positive radiographic finding, consecutively from the mammography screening program at Stockholm South General Hospital. All subjects completed the Headminder Web-based neuropsychological battery Cognitive Stability Index (CSI) for response speed, processing speed, memory, and attention at enrolment (T1, Baseline). CSI was administered again, after BC was ruled out, or after sector resection or mastectomy, if BC was confirmed by cytology or biopsy (T2, Retest). Results and conclusion. Of the 148 women approached, 146 were enrolled; 69 were healthy and 77 had BC. Comparison between groups at baseline, according to independent t-test, showed significant differences in response speed and processing speed. Cognitive abilities did not decline in either group on any of the measured domains. Our results suggest that a diagnosis of BC and subsequent surgery is not associated with substantial cognitive decline at retest. However, the lack of improvement in attention at retest among BC patients may be suggestive of a decline.
Breast cancer (BC) is the most common form of cancer diagnosed in European women today, accounting in 2006 for 429 900 (29%) of all cases of cancer [Citation1]. The effect of BC and its subsequent treatment on cognitive functions remains as an important subject to study, since women receiving adjuvant chemotherapy often report difficulties with memory, attention, and concentration [Citation2,Citation3].
The first investigations of cognitive function in women with BC were cross-sectional studies conducted on women receiving adjuvant chemotherapy after a primary diagnosis of early BC. These studies showed that 16% to 75% of women with BC experienced some degree of cognitive impairment after receiving high doses of standard chemotherapy [Citation4–7] and that some of these effects persisted for up to 10 years after therapy. Aiming to overcome the limitations of cross-sectional studies, such as a lack of baseline testing and little control over confounding factors, longitudinal studies were started to evaluate cognitive performances over time [Citation4,Citation8–10]. In some prospective studies, between 20% and 30% of women with BC had lower cognitive function than in healthy controls, even before starting adjuvant treatment [Citation11,Citation12], mostly in the frontal-subcortical circuitry, affecting memory, attention, processing and response speed [Citation13].
Surgery may have an adverse effect on cognitive functions. Cognitive decline before surgery and after diagnosis has been reported [Citation14]. Women with BC who underwent more invasive surgery for BC exhibited greater cognitive decline than did patients who underwent biopsy only [Citation15]. Postoperative cognitive dysfunction (POCD) has been demonstrated in the early weeks after major non-cardiac surgery, with the elderly being at risk. Studies on regional versus general anaesthesia have not found differences in POCD [Citation16]. Cardiac surgery can be complicated by cognitive decline [Citation17]. Other confounding factors such as depression, anxiety, fatigue and haemoglobin concentration are known to influence neurological ability, therefore they should be taken into account [Citation18].
We started a longitudinal prospective study to investigate the cognitive functions in women who had been screened for BC, before receiving BC diagnosis, after surgery and after adjuvant treatment.
In this report, we sought to determine whether receiving a positive radiographic finding for BC and undergoing subsequent sector resection or mastectomy if BC was confirmed by cytology or biopsy, would affect cognitive functions in women.
Material and methods
The study protocol was approved by the ethics committee of the Karolinska Institute, in compliance with the 1964 World Medical Association's Declaration of Helsinki and the 1975 Tokyo Amendment. Written and verbal informed consent were obtained from all women before enrolment. The first two authors asked the women to participate in all cases.
Study design
In this cohort study, women were recruited from the mammography screening program at the Mammography Department of Stockholm South General Hospital. This screening program includes healthy women living in Stockholm County Council aged 40 to 69 years and in 2006 examined about 800 women weekly. About 20 (2.5%) of these women are recalled each week for further assessment as a result of positive mammographic findings, 10 of whom go on to receive a physical examination by an oncologist or a breast surgeon, and five of whom are eventually diagnosed with BC. The other 10 women, in whom further mammograms of the breast from several angles are negative for BC, continue with the standard mammography screening program.
If a suspicious “spot” is identified on a screening mammogram, the woman is asked to return for further assessment with complementary radiographic views. At this stage, women were invited to participate in our study. The radiologist then decides whether the finding is benign, probably benign, or suspicious for cancer. In the case of a benign finding, women are asked to return at the next scheduled routine screening exam after 18 to 24 months. If the finding is probably benign or suspicious for cancer, the woman is referred to an oncologist or a breast surgeon for further examination and to undergo a fine-needle aspiration cytology or biopsy.
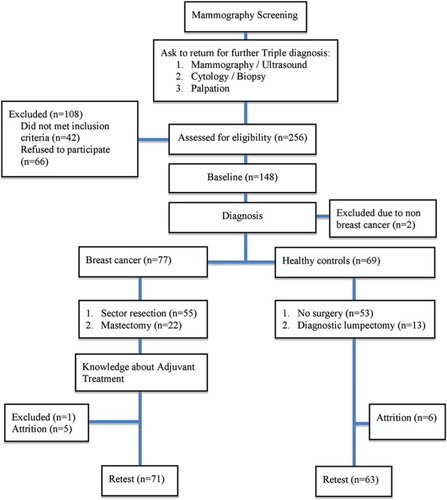
Thus, on the basis of these examinations and biopsies, women were divided into two groups: healthy women, consisting of women whose radiographic findings were eventually determined to be probably benign or suspicious for BC and women whose follow-up biopsies were negative for BC; and women with BC, consisting of women whose radiographic findings were eventually determined to be probably benign or suspicious for BC and whose follow-up biopsies were positive for BC (). Women with a history of malignancy; with a past or current neurological or psychiatric disorder (defined according to International Statistical Classification of Diseases and Related Health Problems); or with a history of chemotherapy, cranial radiotherapy, bone marrow transplantation, or severe head trauma, were excluded.
Testing procedure
The women completed a web-based battery of neuropsychological tests (see below), as well as measures of anxiety, depression, and quality-of-life, in two sessions at the Department of Oncology, Karolinska University Hospital, mostly between noon and 7 pm. The first test session to establish baseline values was just after enrolment, which was prior to diagnosis and the second test session to collect retest values was about two months later for both groups, which was one month after surgery. The women took the test in Swedish, and a trained supervisor was available in the same room to assist them. At each test session, we collected routine blood samples for thyroid function (TSH-, T4-, and T3-levels), haemoglobin count, and menopausal status (follicle-stimulating hormone (FSH) and luteinizing hormone (LH) levels).
Measurements
Assessment of cognitive function. The women completed the Headminder Web-based neuropsychological battery Cognitive Stability Index (CSI), administered in Swedish [Citation19], which provides objective measures of attention, memory, response speed, and processing speed. The CSI was designed to monitor cognitive status in healthy, at-risk, and affected populations. It is designed primarily for use as a longitudinal measurement instrument in prospective studies and consists of a series of repeatable neurocognitive subtests [Citation20]. In total 10 subtests were administered, comprising four cognitive domains (). These four domains have been validated against several traditional neuropsychological tests and have reliable psychometric properties [Citation19]. Response speed correlated with Trail Making A and B (r = 0.73, r = 0.74). Processing speed correlated with Symbol Digit Modalities Test (SDMT) and Symbol Search Test (SST) (r = 0.58, r = 0.65). Memory correlated with Buschke Selective Reminding Test (r = 0.52). Attention correlated with Digit Span (r = 0.62). Test-retest reliability between the first and second assessment ranged from 0.68 to 0.80, with significant practice effects seen only in processing speed [Citation20]. No prior keyboard skills are required. The test takes 30 minutes to complete.
Table I. Neuropsychological variables.
Higher summary scores for attention and memory and lower summary scores for processing speed and response speed indicate higher levels on their respective domains.
Assessment of depression, anxiety, and quality of life. Depression and anxiety were assessed at each test session with the Swedish versions of the Beck Depression Inventory, Second Edition (BDI-II) and the Beck Anxiety Inventory (BAI) [Citation21,Citation22]. The BDI-II questionnaire contains 21 items scored on a scale of 0 to 3. Total scores are interpreted as follows: 0 to 13, no to minimal depression; 14 to 19, mild depression; 20 to 28, moderate depression; and 29 to 63, severe depression. The BAI questionnaire also contains 21 items scored on a scale of 0 to 3. Total scores are interpreted as follows: 0 to 7, minimal anxiety; 8 to 15, mild anxiety; 16 to 25, moderate anxiety; and 26 to 63, severe anxiety. Scale scores on both inventories were analysed as continuous measures. Women with raw scores of 15 or higher on either the BDI-II or the BAI were classified as clinically distressed and those with scores below 15 were classified as normal.
Quality of life was assessed at each test session using the EORTC QLQ C-30 questionnaire, standardised and validated by the European Organisation for Research and Treatment of Cancer Quality-of-Life Group [Citation23]. The QLQ C-30 incorporates five functional scales (physical, role, cognitive, emotional, and social) and nine symptom scales (fatigue, pain, nausea-vomiting, dyspnea, sleep disturbance, appetite loss, constipation, diarrhoea, and financial impact) and one global quality-of-life scale. We used the cognitive function scale to measure the participants’ self-reported cognitive problems. Scores for each scale range between 0 and 100. For scales evaluating global health and function, a higher score represents a higher level of functioning and health. For scales evaluating symptoms, a higher score predicts more symptoms and greater severity.
Statistical methods
The study was designed to compare women recently diagnosed with BC to an equal number of healthy women. We assumed that the incidence of cognitive impairment would be 10% in the healthy women and 30% in the women with cancer. To have 80% power to detect a true difference of 20% between these groups with two-tailed tests at a 5% significance level, we needed to study a sample of 144 women. The rationale for choosing the 20% impairment rate difference between groups is based on previous studies [Citation13].
Baseline characteristics and cognitive-domain-specific raw score were compared between groups with independent two-tailed t-tests and χ2 tests, for continuous and categorical variables, respectively. Mixed linear models (ANCOVA) were used to compare differences between groups after each test measurement. A cognitive-domain-specific raw score was used as a continuous outcome-variable in the analyses. All models controlled for potential confounders, according 1% change of the beta coefficient of the treatment time interaction. The results are shown as least-squares-means for the domain score and 95% confidence intervals from adjusted and unadjusted models. The adjusted covariates were age, education level, pain, B-haemoglobin, depression, and anxiety.
The restricted maximum likelihood, residual degree of freedom [Citation24] and variance components option in the mixed model was used to adjust for random intercept and random slope differences, both at level one and second level [Citation25,Citation26]. All contrast tests were calculated from the final model adjusted with the least-significant-difference method.
Results between groups and between study dropouts and completers (intent-to-treat analysis) were compared with independent two-tailed t-tests and χ2 tests for continuous and categorical variables, respectively.
All data conformed to the assumptions of the statistical tests used to analyse them. A two-sided 5% critical level for a statistical significance was employed. Statistical analyses were performed using SAS version 9.2 for Windows and SPSS version 18 for Mac OS [Citation27].
Results
Patient characteristics
About 256 women were indentified to participate in the study. After screening, 42 women were ineligible on the basis of the exclusion criteria. Of the remaining 214 (84%) women, 66 declined participation mostly due to lack of time and/or lack of interest. Of the 148 women who provided signed consent, two were later excluded from analysis because the findings in the breast were caused by distant metastases from lung cancer and renal cancer. The 146 women analysed consisted of 77 with early BC and 69 healthy women ().
Table II. Characteristics of 146 women tested for cognitive function after notification of a positive mammographic screening test (baseline).
After about two months, 71 women with BC after surgery and 63 healthy women returned to complete the second evaluation. None of the BC women had started any adjuvant treatment at T2. Five women with BC and six healthy women could not return as a result of lack of time, and one woman with BC was withdrawn when she was diagnosed with rheumatoid arthritis. The results indicate that there was no statistical baseline characteristics difference, between the 12 women (8%) who withdrew without completing the second evaluation and the 134 who did.
Confounding factors
Correlation between continuous covariates in all models were controlled by variance-inflation-factor in multiple regression when all covariate were included. The partial correlation between pain and depression after controlling for domain factors shows a positive relationship (R = 0.70). There were no differences between adjusted and unadjusted models concerning domain factors, except in the adjusted model for attention (adjusted p = 0.04 vs. unadjusted p = 0.09).
Effects on cognitive function
Baseline neuropsychological scores. Comparison between groups at baseline, according to independent t-test, shows significant differences in response speed (BC; median 0.71, S.D. 0.11 and healthy controls; median 0.67, S.D. 0.08) and processing speed (BC; median 3.83, S.D. 0.90 and healthy controls; median 3.45, S.D. 0.59).
There was no comparison difference between groups neither in memory nor attention.
Retest neuropsychological scores. Comparison between groups at retest, according to independent t-test, shows significant differences in response speed (BC; median 0.70, S.D. 0.09 and healthy controls; median 0.66, S.D. 0.09), processing speed (BC; median 3.76, S.D. 1.27 and healthy controls; median 3.12, S.D. 0.59) and attention (BC; median 11.02, S.D. 3.45 and healthy controls; median 12.58, S.D. 3.66).
There was no comparison difference between groups in memory.
Change scores between baseline and retest. The aim of the study was to compare and evaluate the effect of BC diagnosis and surgery on cognitive function at retest. An analysis of variance was performed with repeated measures by using the mixed model, which was applied to compare the changes in neuropsychological performance both within and between groups, taking into account the confounding factors.
The random intercept option in the mixed model was used to adjust for heterogeneity differences at baseline on the individual level, both within and between BC patients and healthy controls.
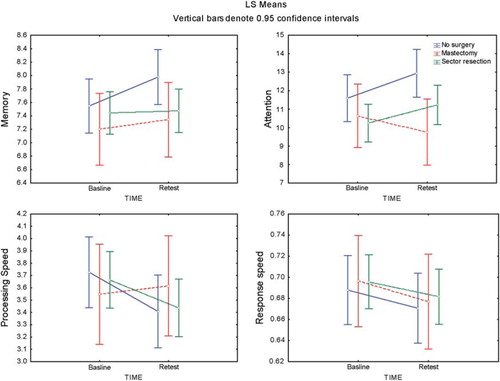
Women recently diagnosed and surgically treated BC showed no changes in any cognitive domains at retest comparing to baseline, in either the adjusted or the unadjusted models.
Healthy women, after diagnosis, improved significantly in attention (p = 0.02) and in processing speed (p < 0.02) at retest comparing to baseline.
The significant difference between groups at retest was shown in attention after adjusting for confounders (p = 0.04) ().
Table III. Least-squares-means for cognitive domain scores for 146 women measured after notification of a positive mammographic screening test (baseline) and again after the diagnosis was ruled out or after sector resection or mastectomy (retest).
Effects of surgery
Of the 77 women with BC, 55 (71%) had sector resection and 22 (29%) had mastectomy with sentinel node dissection, with or without axillary dissection. In addition, 13 healthy subjects (19%) had a diagnostic lumpectomy. All surgery was done with the patients under general anaesthesia. The women's attention and processing speed, after mastectomy, had a negative trend ().
Effects on depression, anxiety, quality of life, and blood samples
Mean BDI-II scores differed between groups, at retest comparing to baseline (p = 0.01). Mean BAI scores improved significantly, at retest comparing to baseline, within both groups (p = 0.05). Mean BDI-II and BAI scores indicate that neither group was distressed at baseline or at retest. Among women with BC, 13 (17%) were distressed at diagnosis (their scores were between 15 and 40) and 15 (21%) were distressed at retest (their scores were between 15 and 35). Among healthy women 14 (20%) were distressed at baseline (their scores were between 15 and 31) and seven (11%) were distressed at retest (their scores were between 15 and 51).
Quality-of-life scores, included participants’ self-reported cognitive problems, measured by the cognitive function scale, did not change significantly at retest comparing to baseline.
We found no correlation between cognitive functions and abnormalities in blood samples (thyroid gland functions, B-haemoglobin count and menopausal status).
Stratified analyses
We also performed stratified analyses to assess possible effect modification according to age (40–50 years, 51–60 years, 61–69 years) and found only significantly improvement in processing speed within healthy controls at age category 40–50 years, at retest comparing to baseline.
Discussion
Previous studies have shown inconsistent results in cognitive function in BC patients. Cognitive decline before initiation of adjuvant treatment has been reported [Citation11,Citation12,Citation14], and the BC diagnosis has been suggested as a potential risk factor for cognitive impairment. Difference between BC patients and controls after surgery but before adjuvant treatment, were not demonstrated in other studies [Citation10,Citation28,Citation29]. Our study is the first, to our knowledge, that evaluated cognitive functioning prior to definitive diagnosis.
Our unadjusted results at baseline suggest a significant difference between the groups in response speed and processing speed, and also in attention at retest. These are inconsistent with our age-stratified results, except in the younger healthy controls, we found better performance in processing speed at retest.
Our results show that neither healthy women nor women with recently diagnosed and surgically treated BC had any cognitive decline in memory, attention, response speed, or processing speed, at retest. Healthy women whose follow-up biopsies were negative for BC, improved significantly at retest in attention and processing speed. Significant interaction in attention was shown between the groups at retest, after adjusting for background factors as covariates. Healthy women improved and BC patients remained stable.
In order to investigate whether more invasive surgery had adverse effect on cognition, we took into account type of surgery in a separate model, since diagnostic lumpectomy was performed among healthy controls. We observed a tendency toward cognitive decline in attention and processing speed in women undergoing more invasive surgery (mastectomy) than in non-operated women and women undergoing less-invasive surgery. However our study was not powered to show this subgroup result and the underlying mechanism is unclear.
Our results showed no relation between cancer diagnosis and cognitive functions, secondary depression, anxiety, or quality of life.
We utilised the Headminder Web-based neuropsychological battery CSI, to measure essential functional domains (response speed, processing speed, memory, and attention). Previous study has shown that CSI is reliable in repeated measures, is brief and has acceptance from cancer patients [Citation30]. The multiple alternate forms of the tests were designed to minimise practice effects. Compared to the traditional neuropsychological test batteries it is time effective and can be implemented in a clinical setting. Further it has less error variance associated with administration, less scoring and examiner bias, and greater precision in measuring reaction time and scoring [Citation30,Citation31].
Practice effects were not corrected for in our analyses. Relatively small improvements in test results at the second session are usually due to knowledge of the test. Lack of improvement on some tasks after repeated testing may be interpreted as a possible indicator for pathology [Citation13,Citation32].
Our results did not show any overall improvement in cognitive functions in BC patients after diagnosis and surgery. The failure to attain an expected practice effect among BC patients may actually be regarded as a possible deficit.
We have found that the performance on the measure of attention significantly improved for healthy women but not for BC patients. This may be due to diagnosis and surgery related stress-response symptoms among BC patients and stress relief among healthy women. Previous longitudinal studies have shown that BC patients after surgery had reduced efficiency in attention and working memory compared to healthy controls [Citation12,Citation33]. BC diagnosis and surgery can trigger traumatic feelings such as threat to life and bodily integrity, experience of disfiguration and disability. Posttraumatic symptoms may be associated with cognitive compromise and have been identified in subgroups of BC survivors [Citation34].
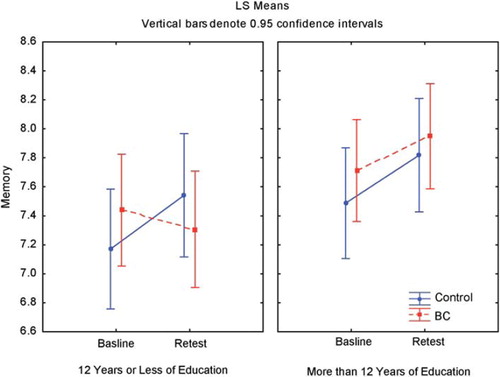
Our sample was divided almost equally between women with high and low levels of education, which is an important predictor of cognitive functions in healthy individuals. Lezak [Citation35] reported that higher education level was related to better performance on objective measures, as did our results, and we found that women with BC and lower education levels showed a greater propensity for cognitive decline, although not statistically significant (). However our study was not powered to show this subgroup result. In a clinical setting, one should consider the impact of lower education level on cognitive functions in women with BC before starting the treatment.
Figure 3. Effect of education on memory among 146 women after notification of a positive mammographic screening test and again after the diagnosis was ruled out or after sector resection or mastectomy.
Another factor with a negative impact on cognitive functions in healthy, cognitively intact, older men and women, is aging [Citation36]. In our study, the mean age of the healthy women was eight years less than that of the BC group (p < 0.01). This difference is explained partly by the County of Stockholm's decision to screen all women aged between 40 and 50 years. This decision was made coincidentally at the start of our study, leading to more re-assessment of healthy women in this age group. Our results show the importance of adjusting for age in these studies.
One limitation of our study was the subject attrition, which is a common problem in longitudinal studies. The attrition rate in our study was 8% across all groups, although there was no baseline characteristics difference on any study variable between women who dropped out and women who remained in the study.
Another limitation was the design of the neuropsychological battery. All domains of cognitive function were not evaluated. In addition, the battery was only 30 minutes in length and did not go into the depth of a traditional neuropsychological battery. Therefore some subtle differences in neuropsychological function may not be apparent.
In conclusion, the present findings suggest that no change in attention in BC patients after diagnosis and surgery can be regarded as decline. We found no published studies that assessed baseline cognitive status before BC diagnosis. We propose that future studies should include baseline testing before receiving the diagnosis. A healthy control group should be included, in this kind of study design. It can provide information about the effects of receiving a benign diagnosis and also the impact of mammography screening on cognitive functions among healthy women.
Further, we found that having a mastectomy and having a lower education level were associated with greater propensity for cognitive decline, which needs to be further investigated. Therefore, it appears that a subgroup of breast cancer patients may be vulnerable to cognitive decline, which is consistent with previous findings [Citation14,Citation15]. Risk factors such as genetic susceptibility, life-style, and environmental exposures may influence the development of cancer and cognitive dysfunction [Citation11].
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ferlay J, Autier P, Boniol M, Heanue M, Colombet M, Boyle P. Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol 2007;18:581–92.
- Shilling V, Jenkins V. Self-reported cognitive problems in women receiving adjuvant therapy for breast cancer. Eur J Oncol Nurs 2007;11:6–15.
- Berglund G, Bolund C, Fornander T, Rutqvist LE, Sjöden PO. Late effects of adjuvant chemotherapy and postoperative radiotherapy on quality of life among breast cancer patients. Eur J Cancer 1991;27:1075–81.
- Wefel JS, Lenzi R, Theriault RL, Davis RN, Meyers CA. The cognitive sequelae of standard-dose adjuvant chemotherapy in women with breast carcinoma. Cancer 2004;100:2292–9.
- Tchen N, Juffs HG, Downie FP, Yi Q-L, Hu H, Chemerynsky I, . Cognitive function, fatigue, and menopausal symptoms in women receiving adjuvant chemotherapy for breast cancer. J Clin Oncol 2003;21:4175–83.
- Wieneke MH, Dienst ER. Neuropsychological assessment of cognitive functioning following chemotherapy for breast cancer. Psycho-Oncology 1995;4:61–6.
- van Dam FS, Schagen SB, Muller MJ, Boogerd W, vd Wall E, Droogleever Fortuyn ME, . Impairment of cognitive function in women receiving adjuvant treatment for high-risk breast cancer: High-dose versus standard-dose chemotherapy. J Natl Cancer Inst 1998;90:210–8.
- Shilling V, Jenkins V, Morris R, Deutsch G, Bloomfield D. The effects of adjuvant chemotherapy on cognition in women with breast cancer – preliminary results of an observational longitudinal study. Breast 2005;14:142–50.
- Fan HG, Houédé-Tchen N, Yi QL, Chemerynsky I, Downie FP, Sabate K, . Fatigue, menopausal symptoms, and cognitive function in women after adjuvant chemotherapy for breast cancer: 1- and 2-year follow-up of a prospective controlled study. J Clin Oncol 2005;23:8025–32.
- Bender CM, Sereika SM, Berga SL, Vogel VG, Brufsky AM, Paraska KK, . Cognitive impairment associated with adjuvant therapy in breast cancer. Psycho-Oncology 2006;15: 422–30.
- Correa DD, Ahles TA. Neurocognitive changes in cancer survivors. Cancer J 2008;14:396–400.
- Cimprich B, Reuter-Lorenz P, Nelson J, Clark PM, Therrien B, Normolle D, . Prechemotherapy alterations in brain function in women with breast cancer. J Clin Exp Neuropsychol 2010;32:324–31.
- Vardy J, Wefel JS, Ahles T, Tannock IF, Schagen SB. Cancer and cancer-therapy related cognitive dysfunction: An international perspective from the Venice cognitive workshop. Ann Oncol 2008;19:623–9.
- Hermelink K, Untch M, Lux MP, Kreienberg R, Beck T, Bauerfeind I, . Cognitive function during neoadjuvant chemotherapy for breast cancer. Cancer 2007;109: 1905–13.
- Wefel JS , Lenzi R, Theriault R, Buzdar AU, Cruickshank S, Meyers CA. ‘Chemobrain’ in breast carcinoma? Cancer 2004; 101:466–75.
- Newman S, Stygall J, Hirani S, Shaefi S, Maze M. Postoperative cognitive dysfunction after noncardiac surgery: A systematic review. Anesthesiology 2007;106:572–90.
- Sauër AM, Kalkman C, van Dijk D. Postoperative cognitive decline. J Anesth 2009;23:256–9.
- Minisini A, Atalay G, Bottomley A, Puglisi F, Piccart M, Biganzoli L. What is the effect of systemic anticancer treatment on cognitive function? Lancet Oncol 2004;5: 273–82.
- Erlanger DM, Kaushik T, Broshek D, Freeman J, Feldman D, Festa J. Development and validation of a web-based screening tool for monitoring cognitive status. J Head Trauma Rehabil 2002;17:458–76.
- Erlanger D, Feldman D, Kutner K, Kaushik T, Kroger H, Festa J, . Development and validation of a web-based neuropsychological test protocol for sports-related return-to-play decision-making. Arch Clin Neuropsychol 2003;18: 293–316.
- Beck AT, Steer RA, Brown GK. Manual for beck depression inventory-II. San Antonio: Psychological Corporation; 1996.
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: Psychometric properties. J Consult Clin Psychol 1988;56:893–7.
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, . The European Organization for Research and Treatment of Cancer qlq-c30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365–76.
- Verbeke G, Molenberghs G. Linear mixed models for longitudinal data. New York: Springer-Verlag New York Inc.; 2009.
- Hedeker D, Gibbons RD. Longitudinal data analysis. New York: John Wiley & Sons Ltd; 2006.
- Leyland AH, Goldstein H. Multilevel modelling of health statistics. Chichester: John Wiley & Sons Ltd; 2001.
- Littell RC, Milliken GA, Stroup WW. SAS for mixed models. 2nd. New York: SAS Publishing; 2006.
- Schagen SB, Muller MJ, Boogerd W, Mellenbergh GJ, van Dam FS. Change in cognitive function after chemotherapy: A prospective longitudinal study in breast cancer patients. J Natl Cancer Inst 2006;98:1742–5.
- Jenkins V, Shilling V, Deutsch G, Bloomfield D, Morris R, Allan S, . A 3-year prospective study of the effects of adjuvant treatments on cognition in women with early stage breast cancer. Br J Cancer 2006;94:828–34.
- Vardy J, Wong K, Yi QL, Park A, Maruff P, Wagner L, . Assessing cognitive function in cancer patients. Support Care Cancer 2006;14:1111–8.
- Gonzalez R, Heaton RK, Moore DJ, Letendre S, Ellis RJ, Wolfson T, . Computerized reaction time battery versus a traditional neuropsychological battery: Detecting hiv-related impairments. J Int Neuropsychol Soc 2003;9:64–71.
- Zehnder AE, Bläsi S, Berres M, Spiegel R, Monsch AU. Lack of practice effects on neuropsychological tests as early cognitive markers of Alzheimer disease? Am J Alzheimers Dis Other Demen 2007;22:416–26.
- Quesnel C, Savard J, Ivers H. Cognitive impairments associated with breast cancer treatments: Results from a longitudinal study. Breast Cancer Res Treat 2009;116:113–23.
- Gurevich M, Devins GM, Rodin GM. Stress response syndromes and cancer: Conceptual and assessment issues. Psychosomatics 2002;43:259–81.
- Lezak MD. Neuropsychological assessment. 4th. New York: Oxford University Press; 2004.
- Braver TS, Barch DM. A theory of cognitive control, aging cognition, and neuromodulation. Neurosci Biobehav Rev 2002; 26:809–17.