Abstract
Background. Patients with recurrent high-grade glioma (HGG) have a poor prognosis and there is no defined standard of care. High levels of vascular endothelial growth factor (VEGF) expressed in HGG make the anti-VEGF monoclonal antibody bevacizumab (BEV) of particular interest. Patients and methods. In an ongoing registry data were collected from patients who have received BEV for the treatment of recurrent HGG. The primary objective was the identification of any clinical benefit as assessed by change in Karnofsky Performance Score (KPS), decreased steroid use and duration of treatment. Results. Two hundred and twenty-five patients with HGG were included (176 glioblastoma; 49 anaplastic glioma; median age 52 years). KPS improved in 10% of patients and remained stable in 68%. Steroids were stopped in 37.6% of patients. Median duration of treatment was 5.5 months; 19.1% of patients were treated for more than 12 months. Median overall survival from beginning of BEV treatment was 8.5 months. At the time of analysis, 169 patients (75.1%) had died and 56 patients (24.9%) were alive. Only 21 patients (9.3%) discontinued treatment due to toxicity. Conclusions. Our data reveal valuable palliation with preservation of KPS and an option for steroid withdrawal in patients treated with BEV, supporting the role of this therapy in late-stage disease.
High-grade gliomas (HGG) account for approximately 23.9% of all primary brain and central nervous system tumours and have an annual incidence rate of 5–7 cases per 100 000 population. The most common type of HGG is glioblastoma (WHO grade IV), accounting for approximately 53.8% of all gliomas [Citation1].
Glioblastoma is associated with poor prognosis, with patients displaying a median survival of 15 months only when treated with current standard of care, involving maximal safe surgical resection followed by radiotherapy with concomitant and adjuvant temozolomide (TMZ) and individual therapy at recurrence [Citation2,Citation3].
Regardless of initial treatment, recurrence of HGG is inevitable. A variety of therapies have been investigated for patients with recurrent disease. According to the NCCN guidelines (www.nccn.org), patients can be offered drugs such as TMZ, nitrosoureas, PCV (procarbazine, CCNU and vincristine), cyclophosphamide, platinum-based regimens or bevacizumab (BEV; Avastin®, Genentech/Roche, Switzerland) alone or in combination with chemotherapy. Second surgery, second courses of radiation therapy and experimental treatment options are also offered at some centres. An overview by the North American Brain Tumour Consortium (NABTC) examined data from 596 patients (159 patients with grade III tumours and 437 patients with grade IV tumours) enrolled in phase II trials for recurrent HGG. Median survival durations, measured from time of registration, were 39 weeks (8.9 months) and 30 weeks (6.9 months) for grade III and grade IV tumours, respectively () [Citation4].
Table I. Common treatment regimens for recurrent glioblastomas.
To date, there is no established standard of care for patients with recurrent HGG. Salvage treatment remains palliative and should therefore meet palliative needs. High levels of vascular endothelial growth factor (VEGF) expression found in HGG [Citation5] have made the VEGF monoclonal antibody BEV of particular interest in therapeutic strategies, both as an adjunct to radiotherapy [Citation6] and in combination with chemotherapy, initially with irinotecan in analogy to previous experiences in colorectal cancer [Citation7–9].
In 2009, the US Food and Drug Administration (FDA) and Swissmedic granted accelerated approval of BEV for the treatment of recurrent glioblastoma, whereas there is currently no such approval in the European Union [Citation10]. The FDA and Swissmedic approval of BEV in recurrent glioblastoma is mainly based on data from two uncontrolled phase II trials [Citation11,Citation12]. The only randomised phase II trial was an open-label, non-comparative, multi-centre trial of BEV alone and in combination with irinotecan in 167 recurrent glioblastoma patients. Median progression-free survival (PFS) was 4.2 months (BEV alone) and 5.6 months (BEV with irinotecan). Median overall survival (OS) was 9.2 months (BEV alone; 95% CI, 8.2–10.7) and 8.7 months (BEV with irinotecan; 95% CI, 7.8–10.9) [Citation11]. The second phase II trial, a single-centre study, investigated single-agent BEV followed by BEV plus irinotecan in 48 heavily pre-treated patients with recurrent glioblastoma. Median PFS was 16 weeks (3.7 months) and median OS was 31 weeks (7.1 months) [Citation12].
Outside of clinical trials, BEV-based regimens are now widely used in several countries where the drug is reimbursed for recurrent malignant glioma.
Our ongoing registry aims at addressing clinical outcome of BEV-based treatment in recurrent HGG after one or multiple prior therapies, using community-based data from patients treated at 30 centres in Switzerland, Austria and Germany.
Methods
In an ongoing and regularly updated registry (starting in August 2006), data on patient characteristics are collected from unselected patients with recurrent HGG treated at 30 centres in Switzerland, Austria and Germany. The decision to treat a patient with recurrent HGG with a BEV-based regimen and the frequency of treatment assessment either radiologically or clinically is the treating physician's choice. Patients are registered at the time of first BEV application, follow-up is until death.
Anonymous data are sampled using an institutional review board-approved written questionnaire. Baseline patient characteristics, medical history regarding the course of HGG, prior treatment regimens and patient outcomes focusing on BEV treatment are recorded.
The main objective of this analysis was the identification of clinical benefit as assessed by change in Karnofsky Performance Score (KPS; baseline KPS and best KPS during BEV-based treatment) and steroid use, as well as duration of treatment. We partitioned KPS into three groups and defined group I as good to excellent (KPS 80–100%) group II as moderate (KPS 60–80%) and group III as poor (KPS < 60%). OS from start of BEV treatment is also of interest; other parameters include reasons for BEV discontinuation and safety. Response rates are not assessed in this cohort due to concerns regarding response criteria for anti-angiogenic treatment and ‘pseudoresponses’, whereby the pseudoresponse reflects a normalisation of abnormally permeable microvessels rather than true tumour shrinkage [Citation13]. In addition, there is a discrepancy between high response rates on conventional radiographic criteria and moderate OS in published phase II trials.
Statistical analysis
Patient characteristics such as gender, age, tumour histology at initial diagnosis and steroid use at the start and during treatment with BEV were assessed. Descriptive statistics using SAS version 8.0 were used to assess: median duration from diagnosis to the date of first BEV treatment administration (days), median duration of BEV therapy (months), KPS before therapy and best score during BEV therapy, and reason for discontinuation of BEV. Time to death after initiation of BEV therapy (days) and time from diagnosis to death (days) were determined using Kaplan–Meier analysis. All analyses are based on a data cut-off point of 31 August 2010.
Results
A total of 225 patients with recurrent HGG were included in this registry [176 patients with glioblastoma and 49 patients with other HGG (anaplastic gliomas of astrocytic, oligodendroglial or mixed phenotypes)]. These diagnoses refer to the histological diagnoses made at first surgery. Patient demographics are shown in . The median age of patients was 52 years (range 19–79). Overall, 20% of patients received BEV monotherapy and 80% were treated with chemotherapy plus BEV. The main combination partner was irinotecan (82% of all combinations), followed by TMZ (9%), lomustine (4.5%) and pegylated liposomal doxorubicin (4.5%). Four of 225 patients received concurrent surgery or irradiation during BEV treatment.
Table II. Patient demographics.
Overall, 40% of patients had a KPS of ≥80% at the start of BEV treatment; 10% of patients showed improvement in their KPS during treatment (from one group to a better group), whereas 9% showed a decline; no data were available from 13% of patients (). No difference was found between WHO grade III and IV tumours in this respect. During treatment, steroids were stopped in 56 of 149 HGG patients (37.6%) who were receiving steroids at baseline ().
Table III. Changes in KPS and corticosteroid use in patients prior to and during BEV.
Median duration of BEV-based treatment (with or without additional chemotherapy) was 5.5 months (range 0.5–39; ). Almost half of the patients (49.3%) were treated for more than six months, 19% for more than one year and 10 patients (4.4%) were treated for more than two years.
Table IV. Duration of BEV treatment.
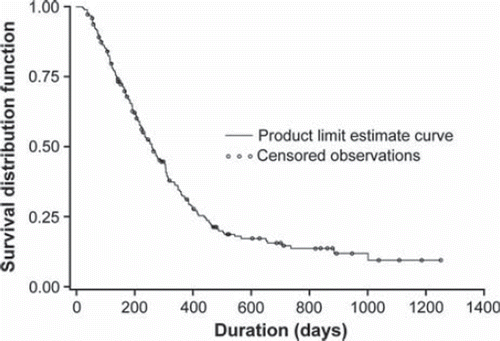
Overall, median OS from beginning of BEV treatment was 8.5 months, with a median OS of 8.3 months in patients with glioblastoma and 9.1 months in patients with other HGG (). A Kaplan–Meier plot of time-to-death analysis from start of BEV treatment is shown in . At the time of this analysis (31 August 2010), 169 patients (75.1%) had died and 56 patients (24.9%) remained alive.
Table V. Overall survival in HGG patients treated with BEV at recurrence.
There was no clear difference in median duration of BEV-based treatment or median OS from beginning of BEV treatment between the following age groups; <50 years, 51–60 years and >60 years (data not shown).
The main reason for discontinuation of BEV treatment was disease progression. Overall, 76% of patients stopped treatment because of clinical or radiological disease progression or both. A total of 21 patients (9.3%) discontinued treatment due to toxicity (). Three fatal CNS bleeding complications and two fatalities due to impaired wound healing accounted for toxic deaths in 2.2% of the patient population. Other treatment-related toxicities, such as hypertension and thromboembolic events, were within the range expected based on phase II trials [Citation11].
Table VI. Reasons for discontinuation of BEV treatment.
Discussion
To date, there is no standard of care in recurrent HGG. Recurrences often become symptomatic with increased intracranial pressure and steroids are regularly the treatment of choice to relieve these symptoms [Citation14]. However, steroids are not generally able to maintain their beneficial effect for a long period of time and can also cause disabling side-effects. In contrast to chemotherapeutic agents available for recurrent HGG, the VEGF antibody BEV has the potential to rapidly lower intracranial pressure by modifying vascular permeability. BEV is a well-tolerated drug, causing severe side-effects in only a low percentage of patients.
Our observational registry of an unselected patient cohort with recurrent and pre-treated HGG from 30 different centres aims to reflect community-based experience with BEV. Evidence for the efficacy of BEV-containing treatment regimens for recurrent HGG stems primarily from unicentric or oligocentric phase II trials. Our experience of a larger and less selected population seems of importance and reproduces benefits for patients with recurrent HGG. Within this patient population only a few patients showed a decline in KPS during BEV treatment. Median duration of treatment was 5.5 months and half of all patients were judged to derive benefit from BEV therapy for at least six months and 19% for at least one year. Median OS of over eight months from the start of BEV treatment for all patients confirms the results from phase II trials. In this cohort of recurrent HGG patients, BEV was given as up to and including the last line of therapy. Therefore, it is not likely that OS was confounded by additional therapeutic approaches given after BEV treatment. However, the analysis of such registry data has limitations, including the possibility of a reporting bias of patients in general and underreporting of adverse events.
Although an often rapid symptomatic improvement with BEV in recurrent HGG is appealing there are some issues that have not yet been resolved, including mechanisms of action and resistance to BEV in HGG, dosing, optimal drug combination and response assessment [Citation13]. Some of these issues will be addressed in a phase II trial from the EORTC Brain Tumour Group which will explore the sequence of BEV and lomustine in glioblastoma patients at first recurrence. In this four-arm study of 249 patients, OS at 12 months will be the primary endpoint (EORTC 26101; www.eortc.be).
In summary, BEV met with the criteria which are claimed for a palliative treatment for HGG patients, that is the preservation of a stable neurological status, withdrawal of steroids and a good tolerability with an acceptable incidence of side-effects. OS confirms findings from phase II clinical trials [Citation11], suggesting that BEV enriches the limited repertoire of medical treatment options for HGG.
Acknowledgements
We sincerely thank Barbara Hofstetter for her invaluable support during the project and critical review of the manuscript. We also thank Laura McDonagh for her editorial support. This work was supported in part by an unrestricted grant from Roche (USZ-NEURO-15122008). SH receives research support and honoraria for advisory board participation and lectures from Roche and Schering Plough. AO is a member of a Roche advisory board. PW is a member of a Schering-Plough advisory board. WW receives lecture honoraria and consultancy honoraria from Roche. MW receives research support and honoraria for advisory board participation and lectures from Merck Serono, Roche and Schering Plough.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Central Brain Tumour Registry of the United States. CBTRUS Statistical Report: Primary brain and central nervous system tumours diagnosed in the United States in 2004–2006. 2010.
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, . Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987–96.
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, . Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 2009;10:459–66.
- Lamborn KR, Yung WK, Chang SM, Wen PY, Cloughesy TF, DeAngelis LM, . Progression-free survival: An important end point in evaluating therapy for recurrent high-grade gliomas. Neuro Oncol 2008;10:162–70.
- Salmaggi A, Eoli M, Frigerio S, Silvani A, Gelati M, Corsini E, . Intracavitary VEGF, bFGF, IL-8, IL-12 levels in primary and recurrent malignant glioma. J Neurooncol 2003; 62:297–303.
- Gutin PH, Iwamoto FM, Beal K, Mohile NA, Karimi S, Hou BL, . Safety and efficacy of bevacizumab with hypofractionated stereotactic irradiation for recurrent malignant gliomas. Int J Radiat Oncol Biol Phys 2009; 75:156–63.
- Desjardins A, Reardon DA, Herndon JE 2nd, Marcello J, Quinn JA, Rich JN, . Bevacizumab plus irinotecan in recurrent WHO grade 3 malignant gliomas. Clin Cancer Res 2008;14:7068–73.
- Vredenburgh JJ, Desjardins A, Herndon JE 2nd, Marcello J, Reardon DA, Quinn JA, . Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol 2007; 25:4722–9.
- Vredenburgh JJ, Desjardins A, Herndon JE 2nd, Dowell JM, Reardon DA, . Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res 2007;13:1253–9.
- Wick W, Weller M, van den BM, Stupp R. Bevacizumab and recurrent malignant gliomas: A European perspective. J Clin Oncol 2010;28:e188–9.
- Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, . Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 2009; 27:4733–40.
- Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I, . Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumour progression in recurrent glioblastoma. J Clin Oncol 2009;27:740–5.
- Dhermain FG, Hau P, Lanfermann H, Jacobs AH, van den Bent MJ. Advanced MRI and PET imaging for assessment of treatment response in patients with gliomas. Lancet Neurol 2010;9:906–20.
- Roth P, Wick W, Weller M. Steroids in neurooncology: Actions, indications, side-effects. Curr Opin Neurol 2010.
- Wick W, Puduvalli VK, Chamberlain MC, van den Bent MJ, Carpentier AF, Cher LM, . Phase III study of enzastaurin compared with lomustine in the treatment of recurrent intracranial glioblastoma. J Clin Oncol 2010;28: 1168–74.
- Wick A, Felsberg J, Steinbach JP, Herrlinger U, Platten M, Blaschke B, . Efficacy and tolerability of temozolomide in an alternating weekly regimen in patients with recurrent glioma. J Clin Oncol 2007; 25:3357–61.