To the Editor,
Several studies have evaluated the ability of 18F-fluorodeoxyglucose (FDG)-positron-emission-tomography/computer tomography (PET/CT) to predict the response to chemotherapy. In patients receiving radiotherapy (RT) or chemo-radiotherapy (CRT) fewer studies have been performed. This has partly been due to concerns regarding RT induced inflammation as a confounder of image analysis [Citation1,Citation2]. Of these studies only a few examine the optimal timing, or early changes in FDG uptake, and even fewer have examined the metabolic changes during therapy with repeated scans.
In patients with pancreatic cancer, data on the efficacy of CRT is conflicting. Randomised studies have shown a lack of effect in the adjuvant and locally advanced setting, while other studies have shown a potential benefit, possibly limited to a certain subset of patients. Prediction of efficacy is for this reason sorely needed.
At our institution we offer CRT to patients with locally advanced pancreatic cancer (LAPC) when a subsequent resection is probable after down-staging. We wanted to improve the outcome of this treatment and hypothesised that a metabolic response in the irradiated tumour might predict efficacy, and potentially select the patients with the highest likelihood of later resection. Lack of studies investigating the optimal timing of FDG-PET/CT scans combined with a clinical interest in the earliest possible prediction, made us perform a pilot series before planning a larger study. All patients had cytological or histological verified adenocarcinoma of the pancreas. Treatment consisted of 50 Gy in 27 fractions concomitant with UFT and leucovorin as previously described [Citation3]. Baseline serology was obtained at the time of treatment planning followed by weekly blood sampling including CA19-9. Within 14 days before CRT patients had a baseline FDG-PET/CT scan. The baseline scan as well as the re-evaluation scan, were performed according to a standard multi-detector-CT pancreas protocol with intravenous iodine contrast, and orally administered water contrast. Monitoring scans were planned during CRT at the 5th, 10th, 15th, 20th, and 27th fraction. These were performed as standard low dose CT-scans (80 mAs, 120kV, 17.5 mm/rotation, slice thickness 3.75 mm). The PET images were acquired using two couch positions over the pancreas and an acquisition time of 2.5 min per field of view. All scans were planned 60 min following the injection of FDG. In selected patients two FDG-PET scans were performed at 60 min and 120 min after the injection (dual-phase). Patients were injected with 4 MBq FDG/kg. All scans were performed in the radiotherapy fixation cast in order to reduce differences in patient movement. Tumour-retention of FDG was estimated from the maximum standardised uptake value (SUVmax) corrected for body weight.
Results
Five patients had a total of 49 FDG-PET/CT scans performed. Patient and disease characteristics are summarised in Supplementary Table 1 (Supplementary Tables to be found online at http://www.informahealthcare.com/doi/abs/10.3109/0284186X.2011.577095). Each patient had seven to 12 of the planned seven to 14 scans performed. Some planned scans were not performed, mostly due to patients being admitted for bile duct occlusions.
It was possible to observe changes in FDG uptake in all patients during CRT. We did not observe inflammation related to the RT in the field (). Patients 2, 3, and 5 experienced infections during CRT; these were followed by an increase in CA19-9 in all three patients as well as an increase in SUVmax in patients 2 and 3. However, large variations in both CA19-9 and SUVmax were observed during CRT in most patients, especially associated with, as mentioned, either biliary occlusions or infectious & bleeding events. Changes in SUVmax as well as changes in CA19-9 are presented in Supplementary Figure 1 (Supplementary Figure 1 to be found online at http://www.informahealthcare.com/doi/abs/10.3109/0284186X.2011.577095).
Figure 1. Illustrate the FDG-PET/CT scans of patient one and five. Patient one did not become resectable after CRT, and progressed with liver metastases detected by EUS at the time of evaluation. Patient 5 became resectable and had a curative resection performed.
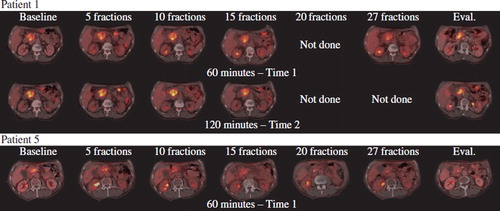
Patient 1, 3, 4 and 5 all showed local tumour response at the time of re-evaluation judged by endoscopic ultrasound. However, patient 1, 2, and 4 had developed liver metastases, while patient 3 had developed peritoneal carcinomatosis detected at an exploratory laparotomy. Patient 5 subsequently underwent a radical resection.
Discussion
To our knowledge this is the first report in pancreatic adenocarcinoma examining the changes in FDG-PET/CT during treatment with CRT. Following recommendations of the EORTC, performing FDG-PET/CT during RT has not been widely attempted, due to concerns of a low signal to noise ratio from RT induced inflammation. This has especially been reported in patients with head/neck and oesophageal cancer; however to what degree this influences response prediction is unknown [Citation2,Citation4]. Presently only smaller studies have examined FDG-PET/CT scan during RT. These studies report results based on a small number of patients, and most perform only a single PET scan during RT. A detailed discussion of these studies is beyond the scope of this letter. In summary the studies show that RT induces some degree of inflammation and gives rise to an increase in SUV, however only few report difficulties in measuring SUV. Supplementary Table 2 gives a short overview of selected studies (Supplementary Tables to be found online at http://www.informahealthcare.com/doi/abs/10.3109/0284186X.2011.577095).
In order to possibly improve the signal to noise ratio we chose to perform dual time scans, since uptake of FDG in tumour tissue has been shown to steadily increase over time [Citation5]. Further; inflamed tissue appear to wash out FDG faster than malignant, and studies with dual-phase FDG-PET in pancreatic cancer have shown that a decline in SUV between an early and late scan is a favourable prognostic marker, and might be used to differentiate between inflamed and malignant tissue [Citation6]. Due to a limited scan capacity we performed dual-phase scans in three of the five patients. These scans showed a considerable variability. Two of the three patients developed bile duct occlusion during CRT, which coincided with an increase in FDG uptake, but in only one of these was an increase in SUVmax at one hour above the SUVmax at two hours, suggesting inflammation. While the patients were admitted for bile duct occlusions awaiting ERCP, chemotherapy but not radiotherapy was paused. Recent results from Janssen et al. in rectal cancer suggest that an early decline in SUVmax is associated with CRT but not RT alone [Citation7]. The variation between fractions could also be explained by a low reproducibility of the estimated SUV-values. However, in a recent study by Nahmias et al. on 26 patients performing repeated FDG-PET-scans with an interval of one to five days, the agreement and correlation between SUVmax as well as SUVmean were excellent [Citation8]. Finally, the variation seen in our and other data, might be explained by the large time-dependant variance in SUVmax observed in our series. We examined whether this could be due to variations in individual waiting times for the patients, but there was no association (data not shown). We can speculate that this variance is due to treatment-induced factors or variations in the scan procedure due to tracer, injection, waiting time, acquisition, reconstruction, processing or maybe even time of the day. Whatever the reason, this variation highlights the need for standardisation of imaging protocols when utilising PET/CT as a predictive marker. Recent publications have stressed this important field [Citation9,Citation10]. In our study the patient that later underwent resection showed a steady decline in SUV during most of the CRT, however three other patients that showed local response had distant progression, proving the aggressiveness of the disease, and the possible difficulties in predicting response to local therapies in this disease.
Conclusion
Performing FDG-PET/CT during CRT for pancreatic adenocarcinoma is feasible. However, inflammation caused by bile-duct occlusion and rapid outside-field progression potentially limits its use as an early predictive marker for local response. Strict standardisation of procedures appears to be needed, especially when it comes to quantitative measures of FDG uptake like the SUVmax.
http://www.informahealthcare.com/doi/abs/10.3109/0284186X.2011.577095
Download PDF (458.5 KB)Acknowledgements
This work was supported by a grant from The Danish Cancer Society. None of the authors who contributed to this work has any conflicts of interest.
References
- Young H, Baum R, Cremerius U, . Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: Review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer 1999;35:1773–82.
- Hentschel M, Appold S, Schreiber A, . Serial FDG-PET on patients with head and neck cancer: Implications for radiation therapy. Int J Radiat Biol 2009;85:796–804.
- Bjerregaard JK, Mortensen MB, Jensen HA, . Long-term results of concurrent radiotherapy and UFT in patients with locally advanced pancreatic cancer. Radiother Oncol 2009;92:226–30.
- Wieder HA, Ott K, Lordick F, . Prediction of tumor response by FDG-PET: Comparison of the accuracy of single and sequential studies in patients with adenocarcinomas of the esophagogastric junction. Eur J Nucl Med Mol Imaging 2007;34:1925–32.
- Beaulieu S, Kinahan P, Tseng J, . SUV varies with time after injection in (18)F-FDG PET of breast cancer: Characterization and method to adjust for time differences. J Nucl Med 2003;44:1044–50.
- Lyshchik A, Higashi T, Nakamoto Y, . Dual-phase 18F-fluoro-2-deoxy-D-glucose positron emission tomography as a prognostic parameter in patients with pancreatic cancer. Eur J Nucl Med Mol Imaging 2005;32:389–97.
- Janssen MH, Ollers MC, van Stiphout RG, . Evaluation of early metabolic responses in rectal cancer during combined radiochemotherapy or radiotherapy alone: Sequential FDG-PET-CT findings. Radiother Oncol 2010.
- Nahmias C, Wahl LM. Reproducibility of standardized uptake value measurements determined by 18F-FDG PET in malignant tumors. J Nucl Med 2008;49:1804–8.
- Boellaard R, Oyen WJ, Hoekstra CJ, . The Netherlands protocol for standardisation and quantification of FDG whole body PET studies in multi-centre trials. Eur J Nucl Med Mol Imaging 2008;35:2320–33.
- Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: Evolving considerations for PET response criteria in solid tumors. J Nucl Med 2009;50(Suppl 1): 122S–150S.
