Abstract
Background. To review the current progress in osteosarcoma stem cells, including isolation and identification, special cell surface markers, relationship between drug-resistance and metastasis, and the involving signal pathways. Methods. A review of the literature encompassing osteosarcoma stem cells was performed. Results. Although the cancer stem cells hypothesis was first proposed about 50 years ago, it is only in the last 10 years that advances in stem cell biology have provided increasing experimental evidence supporting this hypothesis. It has been postulated that within a tumor, a minor subpopulation of cells, termed cancer stem cells (CSC), drive the self-renewal and differentiation that account for the initiation, proliferation, metastasis, therapeutic resistance and recurrence of cancer. The CSC hypothesis opens up a novel conceptual approach for curing tumors that selectively kills CSCs, making it possible to eradicate cancer. Currently, osteosarcoma stem cells have been isolated and identified using various methods. Given the specific stem cell features, the study of CSCs has important implications in osteosarcoma prevention, detection and treatment, especially in curing early metastasis and preventing drug resistance. Focusing on their stem-like character, CSCs can be appropriately targeted by identifying links between the cells and their microenvironment. Conclusion. All of this research is in its infancy – many problems still exist. Further studies are needed to search for specific targeted therapies for osteosarcoma, in-depth study of mechanism of drug resistance, identifying the role that CSCs play in tumor metastasis, and demonstrate the imbalance of specific pathways in osteosarcoma stem cells.
How cancer occurs is a fundamental question in oncology. Classically, there are two conflicting opinions about carcinogenesis, including the clonal evolution model (also called the stochastic model) and the cancer stem cell (CSC) model. In the clonal evolution model, most of the cancer cells possess a tumorigenic potential; some individual cells will undergo additional genetic mutations under certain conditions that promote their malignant behavior, maintain their survival and promote their development. Conversely, the “CSC hypothesis”, which was first proposed by Makino [Citation1] about 50 years ago, postulates that a small subpopulation of cancer cells with unlimited proliferative capacity drive tumor self-renewal and differentiation. There was no substantive progress made in CSC hypothesis until John Dick's team first isolated [Citation2] a subpopulation of cells with CD34(++) CD38(-) phenotype. Apart from the capacity for initiating human acute myeloid leukemia (AML) in non-obese diabetic/mice with severe combined immunodeficiency disease (NOD/SCID mice), this small proportion of cells was demonstrated to possess the differentiation and proliferative capacities and the potential for self-renewal demanded of a leukemic stem cell. From then on, through different methods, researchers have isolated CSCs from many other tumors, including breast cancer [Citation3], central nervous system tumors [Citation4], colon cancer [Citation5,Citation6], prostate cancer [Citation7], pancreatic cancer [Citation8] and hepatic cancer [Citation9]. In addition, CSCs also have been isolated from bone sarcomas using sphere culture systems [Citation10].
Similar to normal stem cells, CSCs have the two fundamental features of stem cells: the capacity for self-renewal and differentiation. Self-renewal is defined as specialized mitotic cell division in which a stem cell creates one (asymmetric) or two (symmetric) daughter stem cells, and differentiation is considered as the overall process of progenitor cells activating genetic and epigenetic mechanisms to define the specialized characteristics of mature cells [Citation11]. These features have been demonstrated in previous studies; i.e. in one study, the CSCs formed tumors in serial transplantation assays and heterogeneous phenotypes different from primary tumors were detected in metastases [Citation12]. Additionally, CSCs and normal stem cells share surface marker phenotypes and molecular machinery related to self-renewal and differentiation [Citation13]. Furthermore, it is likely that CSCs are closely associated with not only carcinogenesis but also metastasis, therapeutic resistance and recurrence of cancer. Osteosarcoma is the most common primary bone malignancy of childhood and adolescence, comprising almost 60% of the common histologic subtypes of bone sarcomas in childhood [Citation14]. Despite use of surgery, radiotherapy and neoadjuvant chemotherapy, long-term survival rates for osteosarcoma have had no significant improvement, stable at approximately 65% [Citation15], attributable to the aggressive malignant potential and early metastasis. All patients with osteosarcoma succumb to death unless they can undergo complete resection of their metastases in case of lung metastasis. CSCs are also implicated in drug resistance [Citation16], another disturbing problem closely linked with poor prognosis.
In studies of osteosarcoma stem cells, the most promising findings concern how stem cells may offer a reasonable explanation of why cancer is so difficult to eradicate and suggest how new therapies might be targeted.
In this review, we will discuss the implications that the CSC hypothesis may have on our basic understanding of osteosarcoma and on the development of new targeted therapies.
The CSC hypothesis and CSC niche
The CSC hypothesis
According to the CSC hypothesis, in the osteosarcoma as well as in other cancers, tumors can be considered as special although abnormal organs composed of a heterogeneous mixture of tumor cells at various levels of differentiation. CSCs exist as a small subpopulation of undifferentiated cells that share properties with their normal tissue-specific stem cell counterparts, including the ability to self-renew, the capacity to generate further differentiated malignant progenitors [Citation17]. In addition, CSCs share similar cell phenotypes (for example, CD34 and CD90, which are pivotal to maintain biological characteristics of stem cells) and similar growth regulatory mechanisms and signal pathways, including Hn, Wnt, Notch, and Hedgehog with normal stem cells.
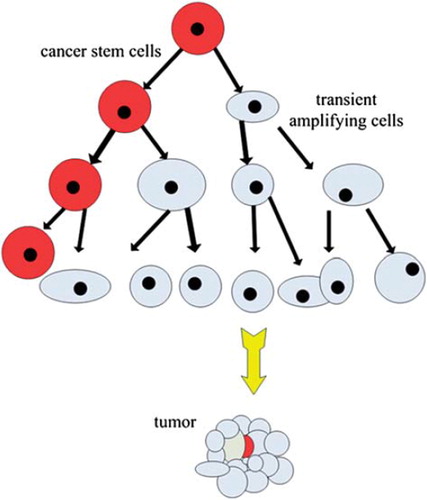
The division model of CSCs is similar to that of normal stem cells. The stem cells divide to produce two daughter cells: an equal one with the self-renewal capacity and a differentiated one responsible for the bulk of cell division, termed a “transient amplifying cell” [Citation18]. In this process, the differentiated daughter cell undergoes genetic changes and epigenetic alterations [Citation19] that make it more tissue-specific but decrease its longevity (). By delegating the dangerous task of repeated gene replication to a dispensable cell (transient amplifying cell), this division model can protect the genome from mutations.
Figure 1. The division model of CSCs: the CSC divide to produce two daughter cells, including an equal one with the self-renewal capacity and a differentiated one responsible for bulk of cells division termed transient amplifying cells. In this process, the transient amplifying cells undergo genetic changes and epigenetic alterations that make it more tissue-specific but shorter lifespan.
Microenvironment (the CSCs niche)
In studies of transplanted hematopoetic progenitors, Schofield [Citation20] first proposed the notion that tissue stem cells reside within specific anatomical locations termed “niches”. Since CSCs are similar to normal stem cells, as mentioned previously, does the “CSC niche” exist? An increasing amount of emerging evidence indicates that factors from the tumor microenvironment play a role in the regulation of cancer cells. Phenomena that change of the surrounding stromal cells lead to neoplastic transformation of epithelial cells have been described in many studies. For instance, tumorigenesis of normal epithelial cells was promoted by irradiation of the extracellular matrix (ECM) [Citation21]. Carcinoma-associated fibroblasts accelerated the carcinogenesis of prostate epithelial cells [Citation22]. Moreover, a visual vascular niche in which brain cancer stem cells (CSC) live was recently described; it is believed that this niche secretes factors to promote the CSC's long-term growth and self-renewal [Citation23]. In AML, the ECM components and signaling molecules in the HSC microenvironment can protect the cancer cells from damage, therefore enhancing the cell survival rate and promoting resistance to chemotherapy [Citation24]. In response to signals from the tumor cells, VEGFR1-expressing bone marrow-derived hematopoietic progenitor cells colonize the premetastatic niche before metastatic tumor cells have arrived to form microenvironment that suitable for CSC, termed “premetastatic niche” [Citation25]. Putting these observations together, we can conclude that the surrounding microenvironment of CSCs indeed greatly affects their growth, self-renewal, drug-resistance, and even invasion and metastasis. Moreover, similarities between the normal stem cell niche and the tumor microenvironment continue to be uncovered; whether there are equivalents of both the quiescent and active niches, as is the case for normal HSCs, remains unknown.
Isolation and identification of osteosarcoma stem cells
The isolation and identification of osteosarcoma stem cells has fundamental implications for further study. How to isolate and identify the small subpopulation of CSCs from tumors was a long-term conundrum that stagnated research with no substantial breakthrough. Since osteosarcoma stem cells were first isolated in anchorage-independent, serum-starved conditions by the use of tumorospheres [Citation10], an increasing number of different methods have been exploited for the purification and isolation of osteosarcoma stem cells. Currently, these methods can be categorized into three main types, including tumorospheres, side population sorting, and sorting cells according to specific cell surface markers.
Tumorospheres
This is a cell culture technique adapted for bone sarcoma tissue based on neurosphere culture techniques, which was used to isolate and expand stem/progenitor cells from normal tissue. Sarcoma stem cells share the features that tend to form sarcospheres in anchorage-independent conditions. Thus, stressful growth conditions were designed for this system, including serum starvation and anchorage-independence, which may select for the most primitive cells by eliminating the differentiated cells that are unable to survive [Citation10]. Sphere culture systems previously have been used to identify CSCs with the capability of self-renewal and tumorigenicity in mouse models [Citation3,Citation4,Citation26,Citation27]. Although it is the most widely used and tested method for isolating osteosarcoma stem cells, sphere culture systems cannot absolutely exclude non-CSCs.
Gibbs [Citation10] first established adherent cultures from biopsies of untreated chondrosarcoma and osteosarcoma, as well as from the commercially available osteosarcoma cell line MG 63. They evaluated each for its ability to generate spherical clones and to self-renew in neurosphere culture system. These monolayer cultures were dissociated into single-cell suspensions and inoculated into a methylcellulose medium without serum, at a clonogenic density of 60 000 cells/well in antiadhesive six-well plates. After 10 to 14 days, all nine initial bone sarcoma cultures and the MG 63 cell line formed spherical colonies, and they found the spherical colonies (“sarcospheres”) forming frequency was 10−2 to 10−3, similar to the reported frequencies in brain and breast malignancies [Citation3,Citation26,Citation28], suggesting an epigenetic mechanism rather than a mutational mechanism. Moreover, follow-up study demonstrated the sarcospheres possess many stem cell characters and perhaps they were osteosarcoma stem cells. Since then, canine OSA cell lines, D-17, UWOS-1 and UWOS-2 [Citation29], Ewing's sarcoma HTB166, fibrosarcoma HT1080 [Citation30], OS99-1, MG63, Hu09 and Saos-2 cells [Citation31] have successfully been isolated using similar methods. Interestingly, in a recent study, CSCs were innovatively isolated and identified by serum-free three-dimensional (3D) culture combined with anticancer drugs from primary cells derived from human osteosarcoma [Citation32].
Sorting cells according to specific surface markers
Combining immunological methods and flow cytometry, this technique sorts the cells with specific surface markers through fluorescence-activated cell sorting (FACS) or magnetic-activated cell sorting (MACS). Subsequently, investigations into self-renewal ability, cell transplantation, clone formation, cell proliferation and cell differentiation were conducted. These results were combined to identify whether the selected cells are CSCs. In spite of the precision of this technique, it is quite difficult to find such specific cell surface markers among the numerous and complex surface makers in the CSCs. Moreover, CSCs share many surface markers with other cells. Despite increasing evidence to support osteosarcoma stem cells, there are not many reports that have successfully demonstrated markers that can be used to identify osteosarcoma stem cells.
CD133, a stem cell marker described for the first time in neuro-endothelial progenitors, was recently proposed as a selective marker for CSCs in some cancers [Citation4,Citation5]. Using the FAC sorting, CSCs were isolated from oteosarcoma SAOS2, MG63 and U2OS by CD133. Subsequent studies confirmed that the sorted CD133 + cells showed stem-like features: a high proliferation rate, cells detected in the G2\M phase of the cell cycle , Ki-67 positivity (a protein expressed only in proliferating cells), and expression of ABCG2 transporters [Citation33]. In addition, CD117 and Stro-1 have been used as specific markers for selecting CSCs in murine and human osteosarcoma. The CD117+ Stro-1+ cells were demonstrated more efficiently to form serially transplantable tumors, that metastasize at a high frequency after orthotopic injections, show high invasiveness and drug-resistant properties and were enrich with CXCR4 (a metastasis-associated marker) and ABCG2 (a drug resistance marker) [Citation34].
Side population cell sorting and other methods
Over expression of transmembrane transporters, such as ATP-binding cassette molecules ABCG2/BCRP1, can make stem cells exclude fluorescent dyes rhodamine 123 and Hoechst 33342, a property that distinguish from differentiated cells. In separating CSCs, the “side population” (SP) cells were defined as a subset of cells that able to exhaust these vital dyes and turn negative. In 2007, Wu et al. [Citation35] first described a side population of cells in osteosarcoma. A recent study found SP cells in NY (0.31%) and MFH2003 (5.28%) (osteosarcoma cell lines), and SP cells from MFH2003 expressed a cancer-initiating ability in vitro and in vivo [Citation36].
Some other interesting methods have also been used in the isolation and identification of osteosarcoma stem cells. Long-term treatment of human osteosarcoma MG-63 cells with 3-aminobenzamide (3AB), a potent inhibitor of poly(ADP-ribose) polymerase (PARP), irreversibly selected novel cancer stem-like cell line (3AB-OS) which was characterized by very high levels of CD133, high levels of ABCG2, nucleostemin, Oct3/4 and Nanog, and a number of other distinctive characteristics that provide these cells with stamina [Citation37]. In another study, Levings et al. [Citation38] identified a functionally distinct, stem-like tumor-initiating cell population by transfecting a tumorigenic osteosarcoma cell line (OS521) with a plasmid in which the human Oct-4 promoter drives expression of green fluorescent protein (GFP).
Stated thus, it seems that we have applied various methods that have been used widely in other tumors to isolate and identify osteosarcoma stem cells. However, we must confess that some of them were reasonably strong in supporting the idea that osteosarcoma stem cells exist, but some seems weak. So far, the most using and promising methods in selecting osteosarcoma stem cells is tumorospheres. Study of cell surface makers, self-renewal capacity, and transplanting test in mouse enriched this method and makes it more credible. Compared with tumorospheres, research of other methods seem relatively few and need further investigation.
There remains a critical problem that whether these laboratories are using different methods to identify the same cells, or are these distinct populations? For example, despite cells expressing stem features had been isolated in osteosarcoma by using sphere culture systems, but we cannot absolutely exclude existence of non-CSCs. In other words, these assumed “osteosarcoma stem cells” perhaps are cell subsets that contain CSCs instead of real osteosarcoma stem cells [Citation39]. It is uncertain whether the Phenomenon of forming sarcospheres in anchorage-independent conditions and capability of self-renewal is caused by penetration of normal stem cells [Citation40]. Judged by excluding fluorescent dyes, the SP cells that possess progenitor cell function were selected. But these SP cell do not equal to osteosarcoma stem cells, and studies using SP cells to identify osteosarcoma stem cells all just declare SP cells have the characteristics of cancer stem-like cells/cancer-initiating cells. The most authoritative method for identifying CSCs is selecting them according to special cell surface markers. Anyway, fundamental prerequisite include the cell surface markers are special enough and all fit cells are CSCs. Although Tirino and Adhikari have primarily isolated cells with stem character by use of CD133, CD117 and Stro-1, these used markers do not satisfy the requirement.
So, unfortunately, we can only consider that osteosarcoma stem cells are very likely to exist and the isolated cells contain CSCs, but there are no direct evidences to prove the selected subset are pure osteosarcoma stem cells. Perhaps, cells isolated using different methods are different. However, it needs further research to compare these cells. The author suggested that further research can combine two or more isolate methods and more homogeneous and accurate osteosarcoma stem cells may be obtained.
Implications in understanding carcinogenesis and osteosarcoma stem cell biology
Features of osteosarcoma stem cells
As mentioned previously, stem cells have two main features, self-renewal and multilineage differentiation, which are shared by osteosarcoma stem cells. The CSCs could form sarcospheres in serum starvation and anchorage-independent conditions, whereas the non-CSCs could not. When dissociated to single cells and allowed to grow in monolayers, CSCs demonstrated self-renewal through the formation of secondary spheres at a similar or increased frequency. To test tumor-initiating capacity in vivo, CSCs were orthotopically injected into the femoral bone marrow space of anesthetized NOD/SCID mice, and the transplantated tumor grew and even metastasized [Citation38]. Furthermore, cells derived from osteosarcoma can be induced to differentiate along at least two mesenchymal lineages [Citation10]. Synthesizing these observations, CSCs indeed possess stem cells characteristics.
Simultaneously, by using semiquantitative reverse transcriptase polymerase chain reaction (RT-PCR) analyses, western blot and immunohistochemical analyses, it has been confirmed that osteosarcoma stem cells share markers with other stem cells. Osteosarcoma stem cells express the mesenchymal stem cell markers Stro-1, CD44, and CD105 [Citation10,Citation34,Citation38], and preferentially express key marker genes of ES cell pluripotency, including Oct3/4 , Nanog, Stat3 and Sox2 [Citation10,Citation29–32,Citation37]. Moreover, osteosarcoma stem cells also express high levels of CD133, CD117, ABCG2, CXCR4, ICAM-1 and nucleostemin [Citation33,Citation34,Citation37,Citation38], which have been considered to be specific markers. Oct3/4 expression is believed to play a vital role in tumorigenesis. However, Oct3/4 expression studies on tumors are generally carried out without considering isoforms. Since the existence of two mRNA, protein isoforms of Oct3/4 (Oct3/4A and Oct3/4B) has been validated [Citation41], Wang et al. [Citation31] examined these Oct3/4 isoforms in osteosarcoma for the first time. In their study, by using RT-PCR and flow cytometry, they demonstrated Oct3/4A expression was significantly upregulated in the OS99-1, Hu09 and MG63 cells compared with the Saos-2 cells, suggesting that lower Oct3/4A expression may be seen in non-tumorigenic cells since Saos-2 is a non-tumorigenic cell line while others are tumorigenic cell lines. Interestingly, expression of Oct3/4B in the Hu09 cell line was significantly higher than in OS99-1, Saos-2 and MG63 cell lines. The higher expression of Oct3/4B noted in the Hu09 cell line may reflect its aggressiveness since this human osteosarcoma cell line is known to have a high rate of metastasis to lung in nude mice after intravenous injection [Citation42]. So, one could come to the conclusion that the stemness property of Oct3/4 may assigned to Oct3/4A which functions as a master switch during differentiation by regulating the pluripotent potential. And perhaps, Oct3/4 B was associated with metastasis.
Implications in the metastasis of cancer
Metastasis is a complex and multistep process in which cancer cells spread from primary tumor sites to distant organs and tissues then proliferate to form new metastatic lesions, accounting for over 90% of the deaths in cancer patients [Citation43]. There are two conflicting hypothesis about metastasis. The clonal selection theory holds that a subpopulation of tumor cells acquire metastatic ability through gene mutation in the late stage of tumor development, then transfer to other sites in body to form metastatic lesions. However, other theories suggest that there are a small proportion of cells termed “ mCSCs ” that acquired metastatic ability in the initial stage of tumor development [Citation44]. Induced by specific signals, these cells will transfer to a new microenvironment and proliferate to form new metastatic lesions [Citation25,Citation45].
Two indispensable factors in cancer metastasis are the seeding and colonization of specialized cells. CSCs have been shown as the seeds in studies of breast cancer that demonstrated that CSCs are programed to preferentially metastasize to specific organs [Citation25,Citation46,Citation47]. Moreover, all types of cancers are prone to transfer to several fixed organs or tissues termed “tissue tropisms” of metastasis, this theory indicates that specific and distinct cellular and molecular mechanisms may be involved. For instance, the most likely site of metastases for osteosarcoma is the lung. If this theory is valid, what are the cellular and molecular mechanisms involved? Li and colleagues proposed a metastasis model based on CSCs. During establishment of the CSC pool, CSCs inherit a unique set of genetic and/or epigenetic changes that determines the cancer malignancy, metastatic potential and the tissue tropism. The pre-metastasis niches, in which the metastases will form, appear before the metastasis. By secreting stimulatory signals, the pre-metastasis niche makes contact with the primary tumor and helps govern the homing of mCSCs. Cues, such as oxygen gradients or other chemo-attractants, derived from niche sites guide mCSC trafficking toward preferred tissues and organs [Citation45]. To form organ-specific metastases, the mCSCs not only must adapt to a particular target tissue microenvironment but also need to adjust the microenvironment to become more suitable for the survival of their progeny.
CXCR4, a chemokine receptor in the GPCR gene family, has been proven to play essential role in the metastasis of mCSCs [Citation48]. In fact, it also has been reported that both primary tumor formation and metastasis were effectively inhibited once the homing factor CXCR4 was blocked in animal models [Citation49]. Applying flow cytometry, osteosarcoma stem cells were found to express more CXCR4 than normal tumor cells [Citation34].
Fundamental implications for osteosarcoma management
Current therapies for osteosarcoma, including surgery, various types of chemotherapy including neoadjuvant chemotherapy and radiotherapy, all will kill healthy cells while fighting cancer cells, causing side effects that cannot be ignored. In spite of great improvements in therapeutic strategies and outcomes, troubling problems, including therapeutic resistance, cancer recurrence and the lack of curative treatment in metastatic disease, indicate that eradicating osteosarcoma may be a long way away.
Osteosarcoma stem cells and drug resistance
Intrinsic or acquired tumor drug resistance is closely related to recurrence. Increasing evidence has emerged to substantiate that CSCs are involved in mechanisms of drug resistance [Citation50–52], indicating that to solve the problem of tumor recurrence one must first eliminate this subpopulation of cells.
In spite of the fact that the specific role that CSCs play in the mechanism of drug-resistance has not been clearly elucidated, there are several possible mechanisms that could mediate intrinsic drug-resistance in CSC [Citation53]. First, the cell cycle in CSCs is generally less active and is relatively quiescent compared with that in other cells, although this appears to be debatable in osteosarcoma [Citation33,Citation54,Citation55]; this mechanism needs further confirmation. Second, CSCs have a strong drug-efflux system. Certain ABC multidrug efflux transporters, including P-glycoprotein (P-gp), the multidrug resistance associated protein 1 (MDR1) and BCRP/ABCG2 products, are frequently overexpressed in CSCs. By using immunofluorescence microscopy, the osteosarcoma stem cells were observed to express more of the stem cells marker ABCG2 than normal tumor cells, and their efflux capacity cannot even be affected by verapamil, the inhibitor of ABCB1 [Citation33,Citation34]. Third, chemotherapeutic agents generally cause DNA damage or interfere with cellular metabolism, resulting in apoptosis when DNA damage is critical or DNA repair is inefficient. Therefore, the efficiency of DNA mismatch repair in CSC could enhance their resistance to chemotherapeutic treatments. The formed sarcospheres express strong resistance to doxorubicin and cisplatin, the effect which can be increased by caffeine, a DNA repair inhibitor. In addition, the expression of the DNA repair gene (MLH1 and MSH2) is increased [Citation30]. All of these indicate that osteosarcoma drug resistance was related to the expression of DNA repair enzymes and DNA repair efficiency. Fourth, CSCs possess corresponding detoxification mechanisms; aldehyde dehydrogenase-1 (ALDH1) which plays a large role in drug detoxification has been shown to confer resistance to cyclophosphamide in normal and malignant stem cells [Citation56]. The subpopulation cells with elevated ALDH1 share stem-like features, and possess chemo-resistant properties in osteosarcoma MG63 [Citation57]. Finally, resistance to apoptosis is enhanced; osteosarcoma stem cells express more anti-apoptotic proteins, including Bcl-2, FLIP, apoptosis inhibitor XIAP, IAP-1, IAP-2 and survivin, than normal osteosarcoma cells [Citation37]. Fujii et al. [Citation12] analyzed sarcospheres from rat osteosarcoma and malignant fibrous histiocytoma cell lines that developed in anchorage-independent, serum-starved conditions. Spheres from both sarcomas remarkably decreased the expression of INK4a/ARF locus genes p16INK4a and p19ARF, which could be related to the resistance against cell senescence and apoptosis. Therefore, subpopulations could evade senescence and apoptosis through the p53 and Rb pathways to resist toxicity of chemotherapy.
Specific pathways
It is believed that stem cells self-renew and differentiation, control of the pool of stem cells is driven by some specific signaling pathways. Therapy targeting these pathways may affect the behavior of the CSCs, thus deciding the outcome of tumor.
The hedgehog (HH) pathway is one of the main pathways that drive stem cell fate, self-renewal and maintenance [Citation58]. A recent study has demonstrated this pathway is essential in controlling the behavior of glioma stem cells, representing a novel therapeutic target [Citation59]. Present study found that Shh, Dhh, PTCH1, SMO, GLI1 and GLI2 transcripts were over-expressed in osteosarcoma cell line. In general, it is accepted that enhanced HH pathway activation leads to downstream expression of target genes including PTCH1 and GLI, and hence, the levels of these transcripts are often used as surrogate markers of HH pathway activity [Citation60]. Thus, these findings show HH pathway is activated in osteosarcomas and cyclopamine can prevent osteosarcoma growth by cell cycle regulation [Citation61]. Research about the NOTCH pathway has found that inhibitors of the γ-secretase complex deplete stem cells and slow the growth of NOTCH-dependent tumors [Citation62,Citation63], which is in accordance with a study that showed the NOTCH pathway is activated in osteosarcoma and inhibitors of γ-secretase inhibit osteosarcoma growth by cell cycle regulation [Citation64]. Another focused pathway is the Wnt/β-catenin pathway, which is often inactive in conventional high-grade osteosarcoma [Citation65]. Interestingly, by acting through the Wnt/β-catenin pathway, CD99 could inhibit osteosarcoma malignacy [Citation66].
In addition to pathways like HH, NOTCH and Wnt/β-catenin confirmed to play a large role in many types of CSCs, the MAPK pathway have also been observed to play an important role in the pathogenesis of osteosarcoma. ERK, JNK and p38, components of MAPK pathway, form an intercoordinating network and regulate cell proliferation, differentiation, apoptosis, invasion and migration in osteosarcoma [Citation67]. In addition, Arsenic trioxide has been proven to inhibit osteosarcoma cell invasiveness via the MAPK signaling pathway [Citation68]. Other pathways linked to osteosarcoma stem cells include Fas/FasL, Transcription 3 (Stat3) among others.
Many different pathways could be involved in determining the fate of osteosarcoma stem cells. Recent study about mammary stem cells has showed not only HH signaling pathway were activated in breast cancer but also activation of HH signaling increases the number of mammospheres-initiating cells and mammosphere size, whereas inhibition of the pathway results in a reduction of these parameters [Citation69]. Although changes of above signaling pathways in osteosarcoma have not been demonstrated in CSCs level as that in breast cancer, the commonly accepted idea holds that all these changes are helpful for maintaining infinitely self-renewal and proliferation, and these changes are different in normal osteoblasts, differentiated osteosarcoma cells, and osteosarcoma stem cells. For instance, HH signaling pathway are more active in differentiated osteosarcoma cells and osteosarcoma stem cells than that in normal osteoblasts, but changes in osteosarcoma stem cells are more significant. Due to the need of stem features, the signaling pathway changes in CSCs are always the most outstanding varieties. Inhibiting these signaling pathways might greatly effect growth and metabolism of CSCs, however, little to normal cells. All these information highlights the potential of developing a novel anticancer therapy based on blocking the relative signaling pathways.
For treatment of CSCs, induced differentiation is also a viable choice. Examples of this method include curing acute promyelocytic leukemia using all-trans retinoic acid, which has demonstrated a significant clinical effect [Citation70]. During this process, all-trans retinoic acid may have induced the differentiation of leukemia stem cells. In osteosarcoma, searching for the appropriate differentiation-inducing drugs also may be a novel topic for future research. For instance, on treatment with the PPARγ and RXR ligands, osteosarcoma lines exhibited a significantly reduced proliferation rate and cell viability, in addition, activity of alkaline phosphatase, a well-characterized hallmark for osteoblastic differentiation, were observed to be effectively induced. All these findings suggest nuclear receptor agonists as potential differentiation therapy agents for human osteosarcoma [Citation71].
The CSC hypothesis involves a novel understanding that CSCs are responsible for tumorigenesis, tumor development, metastasis, and drug resistance. Based on this understanding, the strategy with which we treat osteosarcoma will also be updated. Through in-depth study of biological characteristics and related signal pathways to identify specific targets, that target the regulatory mechanism of CSCs, developing selective toxic drugs for CSCs offers hope for eradicating osteosarcoma.
Conclusion
Despite doubts, the CSC hypothesis is very attractive for its potential implications in eradicating cancers by targeting and eliminating CSCs. The CSC hypothesis modifies our understanding of osteosarcoma oncogenesis and provides rational support for classification, detection and prevention. Currently, osteosarcoma stem cells have been isolated and identified by various methods, including tumorospheres, sorting cells according to specific surface markers, side population cells sorting and other methods. The subpopulation cells express more stem cell markers, such as Oct3/4 and Nanog, than osteosarcoma cells. Additionally, the CSCs express strong tumorogenic capacity and can even form lung metastasis. More and more studies have revealed implications for osteosarcoma stem cells in the initiation of cancer and its metastasis and drug resistance. Many different pathways, including HH, NOTCH, Wnt/β-catenin and MAPK, could be involved in the determination of the fate of osteosarcoma stem cells. However, all of this research is in its infancy. Still, many problems exist. For instance, no one has been able to completely eliminate non-CSCs, no matter which method is used for isolation and identification of osteosarcoma stem cells. Mechanisms of CSC self-renewal, regulation and metastasis need further systemic study. The specific mechanism of osteosarcoma stem cells drug resistance has not yet been clearly revealed.
Further studies are needed to search for specific targeted therapies for osteosarcoma, in-depth study of mechanism of drug resistance, identifying the role that CSCs play in tumor metastasis, and demonstrate the imbalance of specific pathways in osteosarcoma stem cells. The fact that the stem cell niche plays an essential role in deciding the stem cell's fate and resistance to therapeutic drugs should be considered in developing new treatment strategies [Citation72].
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Makino S. The role of tumor stem-cells in regrowth of the tumor following drastic applications. Acta Unio Internationalis Contra Cancrum 1959;15:196–8.
- Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med 1997;3:730–7.
- Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA 2003;100:3983–8.
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, . Identification of human brain tumour initiating cells. Nature 2004;432(7015):396–401.
- Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, . Identification and expansion of human colon-cancer-initiating cells. Nature 2007;445(7123):111–5.
- O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 2007;445(7123):106–10.
- Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res 2005;65:10946–51.
- Li CW, Heidt DG, Dalerba P, Burant CF, Zhang LJ, Adsay V, . Identification of pancreatic cancer stem cells. Cancer Res 2007;67:1030–7.
- Yang ZF, Ho DW, Ng MN, Lau CK, Yu WC, Ngai P, . Significance of CD90(+) cancer stem cells in human liver cancer. Cancer Cell 2008;13:153–66.
- Gibbs CP, Kukekov VG, Reith JD, Tchigrinova O, Suslov ON, Scott EW, . Stem-like cells in bone sarcomas: Implications for tumorigenesis. Neoplasia 2005;7:967–76.
- Lobo NA, Shimono Y, Qian D, Clarke MF. The biology of cancer stem cells. Ann Rev Cell Dev Biol 2007;23:675–99.
- Fujii H, Honoki K, Tsujiuchi T, Kido A, Yoshitani K, Mori T, . Reduced expression of INK4a/ARF genes in stem-like sphere cells from rat sarcomas. Biochem Biophys Res Comm 2007;362:773–8.
- Shackleton M. Normal stem cells and cancer stem cells: Similar and different. Semin Cancer Biol 2010;20:85–92.
- Gatta G, Capocaccia R, Stiller C, Kaatsch P, Berrino F, Terenziani M. Childhood Cancer Survival Trends in Europe: A EUROCARE Working Group Study. J Clin Oncol 2005;23:3742–51.
- Meyers PA, Krailo M, Grier H, Bernstein M. Prospectively planned analysis of data from a phase III study of liposomal muramyltripeptide phosphatidylethanolamine in the treatment of osteosarcoma – Reply. J Clin Oncol 2005;23:6438–9.
- Honoki K. Do stem-like cells play a role in drug resistance of sarcomas? Expert Rev Anticancer Ther 2010;10:261–70.
- Wicha MS, Liu SL, Dontu G. Cancer stem cells: An old idea – A paradigm shift. Cancer Res 2006;66:1883–90.
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature 2001;414(6859): 105–11.
- Matsunaga T, Takemoto N, Sato T, Takimoto R, Tanaka I, Fujimi A, . Interaction between leukemic-cell VLA-4 and stromal fibronectin is a decisive factor for minimal residual disease of acute myelogenous leukemia. Nat Med 2003;9: 1158–65.
- Schofield R. Relationship between spleen colony-forming cell and hematopoietic stem-cell-hypothesis. Blood Cells 1978;4:7–25.
- Chang PY, Bjornstad KA, Chang E, McNamara M, Barcellos-Hoff MH, Lin SP, . Particle irradiation induces FGF2 expression in normal human lens cells. Radiat Res 2000;154:477–84.
- Tisty T, Grossfeld GD, Hayward SW, Olumi AF, Cunha GR, Carroll PR. Carcinoma-associated fibroblasts stimulate tumor progression of initiated human prostatic epithelium. Mol Biol Cell 1999;10:351a–a.
- Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, . A perivascular niche for brain tumor stem cells. Cancer Cell 2007;11:69–82.
- De Toni F, Racaud-Sultan C, Chicanne G, Mansat-De Mas V, Cariven C, Mesange F, . A crosstalk between the Wnt and the adhesion-dependent signaling pathways governs the chemosensitivity of acute myeloid leukemia. Oncogene 2006;25:3113–22.
- Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, . VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 2005;438(7069):820–7.
- Hemmati HD, Nakano I, Lazareff JA, Masterman-Smith M, Geschwind DH, Bronner-Fraser M, . Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci USA 2003;100:15178–83.
- Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, . Identification of a cancer stem cell in human brain tumors. Cancer Res 2003;63:5821–8.
- Ignatova TN, Kukekov VG, Laywell ED, Suslov ON, Vrionis FD, Steindler DA. Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro. Glia 2002;39:193–206.
- Wilson H, Huelsmeyer M, Chun R, Young KM, Friedrichs K, Argyle DJ. Isolation and characterisation of cancer stem cells from canine osteosarcoma. Vet J 2008;175:69–75.
- Fujii H, Honoki K, Tsujiuchi T, Kido A, Yoshitani K, Takakura Y. Sphere-forming stem-like cell populations with drug resistance in human sarcoma cell lines. Int J Oncol 2009;34: 1381–6.
- Wang L, Park P, Lin CY. Characterization of stem cell attributes in human osteosarcoma cell lines. Cancer Biol Ther 2009;8:543–52.
- Zhou S. Isolation and identification of cancer stem cells from human osteosarcom by serum-free three-dimensional culture combined with anticancer drugs. J Huazhong Univ Sei Technol (Med Sci) 2010;30:81–4.
- Tirino V, Desiderio V, d'Aquino R, De Francesco F, Pirozzi G, Galderisi U, . Detection and characterization of CD133(+) cancer stem cells in human solid tumours. Plos One 2008;3:e3469.
- Adhikari AS, Agarwal N, Wood BM, Porretta C, Ruiz B, Pochampally RR, . CD117 and Stro-1 identify osteosarcoma tumor-initiating cells associated with metastasis and drug resistance. Cancer Res 2010;70:4602–12.
- Wu CL, Wei QX, Utomo V, Nadesan P, Whetstone H, Kandel R, . Side population cells isolated from mesenchymal neoplasms have tumor initiating potential. Cancer Res 2007;67:8216–22.
- Murase M, Kano M, Tsukahara T, Takahashi A, Torigoe T, Kawaguchi S, . Side population cells have the characteristics of cancer stem-like cells/cancer-initiating cells in bone sarcomas. Br J Cancer 2009;101:1425–32.
- Di Fiore R, Santulli A, Ferrante RD, Giuliano M, De Blasio A, Messina C, . Identification and expansion of human osteosarcoma-cancer-stem cells by long-term 3-aminobenzamide treatment. J Cell Physiol 2009;219:301–13.
- Levings PP, McGarry SV, Currie TP, Nickerson DM, McClellan S, Ghivizzani SC, . Expression of an exogenous human Oct-4 promoter identifies tumor-initiating cells in osteosarcoma. Cancer Res 2009;69:5648–55.
- Li HZ, Yi TB, Wu ZY. Suspension culture combined with chemotherapeutic agents for sorting of breast cancer stem cells. BMC Cancer 2008;8:135.
- Reynolds BA, Rietze RL. Neural stem cells and neurospheres – re-evaluating the relationship. Nat Methods 2005;2:333–6.
- Kotoula V, Papamichos SI, Lambropoulos AF. Revisiting OCT4 expression in peripheral blood mononuclear cells. Stem Cells 2008;26:290–1.
- Gillette JM, Gibbs CP, Nielsen-Preiss SM. Establishment and characterization of OS 99-1, a cell line derived from a highly aggressive primary human osteosarcoma. In Vitro Cell Dev Biol–Animal 2008;44:87–95.
- Weigelt B, Peterse JL, van't Veer LJ. Breast cancer metastasis: Markers and models. Nature Rev Cancer 2005;5:591–602.
- Bernards R, Weinberg RA. Metastasis genes: A progression puzzle. Nature 2002;418(6900):823.
- Li F, Tiede B, Massague J, Kang YB. Beyond tumorigenesis: Cancer stem cells in metastasis. Cell Res 2007;17:3–14.
- Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu WP, Giri DD, . Genes that mediate breast cancer metastasis to lung. Nature 2005;436(7050):518–24.
- Minn AJ, Kang YB, Serganova I, Gupta GP, Giri DD, Doubrovin M, . Distinct organ-specific metastatic potential of individual breast cancer cells and primary tumors. J Clin Invest 2005;115:44–55.
- Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, . Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell 2007;1:313–23.
- Smith MCP, Luker KE, Garbow JR, Prior JL, Jackson E, Piwnica-Worms D, . CXCR4 regulates growth of both primary and metastatic breast cancer. Cancer Res 2004;64:8604–12.
- Beachy PA, Karhadkar SS, Berman DM. Tissue repair and stem cell renewal in carcinogenesis. Nature 2004; 432(7015):324–31.
- Ravandi F, Burnett AK, Agura ED, Kantarjian HM. Progress in the treatment of acute myeloid leukemia. Cancer 2007;110:1900–10.
- Woodward WA, Sulman EP. Cancer stem cells: Markers or biomarkers? Cancer Metastasis Rev 2008;27:459–70.
- Honoki K. Do stem-like cells play a role in drug resistance of sarcomas? Expert Rev Anticancer Ther 2010;10:261–70.
- Song B, Wang YA, Titmus MA, Botchkina G, Formentini A, Kornmann M, . Molecular mechanism of chemoresistance by miR-215 in osteosarcoma and colon cancer cells. Molecular Cancer 2010;9:96.
- Song B, Wang Y, Xi Y, Kudo K, Bruheim S, Botchkina GI, . Mechanism of chemoresistance mediated by miR-140 in human osteosarcoma and colon cancer cells. Oncogene 2009;28:4065–74.
- Gangemi R, Paleari L, Orengo AM, Cesario A, Chessa L, Ferrini S, . Cancer stem cells: A new paradigm for understanding tumor growth and progression and drug resistance. Curr Med Chem 2009;16:1688–703.
- Honoki K, Fujii H, Kubo A, Kido A, Mori T, Tanaka Y, . Possible involvement of stem-like populations with elevated ALDH1 in sarcomas for chemotherapeutic drug resistance. Oncol Report 2010;24:501–5.
- McMahon AP. More surprises in the Hedgehog signaling pathway. Cell 2000;100:185–8.
- Clement V, Sanchez P, de Tribolet N, Radovanovic I, Altaba ARI. HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr Biol 2007;17:165–72.
- Watkins DN, Peacock CD. Hedgehog signalling in foregut malignancy. Biochem Pharmacol 2004;68:1055–60.
- Hirotsu M, Setoguchi T, Sasaki H, Komiya S. Hedgehog pathway is activated in osteosarcoma and cyclopamine prevents osteosarcoma growth by cell cycle regulation. J Bone Mineral Res 2008;23:301–7.
- Fan X, Matsui W, Khaki L, Stearns D, Chun J, Li YM, . Notch pathway inhibition depletes stem-like cells and blocks engraftment in embryonal brain tumors. Cancer Res 2006;66:7445–52.
- Weng AP, Millholland JM, Yashiro-Ohtani Y, Arcangeli ML, Lau A, Wai C, . c-Myc is an important direct target of Notch1 in T-cell acute lymphoblastic leukemia/lymphoma. Genes Dev 2006;20:2096–109.
- Tanaka M, Setoguchi T, Hirotsu M, Sasaki H, Komiya S. Notch pathway is activated in osteosarcoma and inhibitors of gamma-secretase inhibit osteosarcoma growth by cell cycle regulation. J Bone Mineral Res 2008;23:297–301.
- Cai YP, Mohseny AB, Karperien M, Hogendoom PCW, Zhou GY, Cleton-Jansen AM. Inactive Wnt/beta-catenin pathway in conventional high-grade osteosarcoma. J Pathol 2010;220:24–33.
- Sciandra M, Barbieri S, Picci P, Scotlandi K. CD99 inhibits osteosarcoma malignacy by acting through Wnt pathway. Proc Am Assoc Cancer Res Annual Meeting 2009;50:845.
- Li G-D, Cai Z-D, Zhang Y-Q, Gong H-Y, Tang H, Zhang Q-L. Gene profiling of MAPK pathway in human osteosarcoma. Zhonghua Zhongliu Zazhi 2009;31:340–5.
- Ren TT, Guo W, Peng CL, Lu XC, Yang-Yi. Arsenic trioxide inhibits osteosarcoma cell invasiveness via MAPK signaling pathway. Cancer Biol Ther 2010;10:251–7.
- Liu SL, Dontu G, Mantle ID, Patel S, Ahn NS, Jackson KW, . Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res 2006;66:6063–71.
- Chen ZX, Xue YQ, Zhang R, Tao RF, Xia XM, Li C, . A clinical and experimental-study on all-trans retinoic acid-treated acute promyelocytic leukemia patients. Blood 1991;78:1413–9.
- Haydon RC, Zhou L, Feng T, Breyer B, Cheng HW, Jiang W, . Nuclear receptor agonists as potential differentiation therapy agents for human osteosarcoma. Clin Cancer Res 2002;8:1288–94.
- Charafe-Jauffret E, Monville F, Ginestier C, Dontu G, Birnbaum D, Wicha MS. Cancer stem cells in breast: Current opinion and future challenges. Pathobiology 2008; 75:75–84.
