Abstract
Background. Carbon ion (12C) therapy in the treatment of prostate cancer (PC) might result in an improved outcome as compared to low linear energy transfer irradiation techniques. In this study, we present the first interim report of acute side effects of the first intermediate-risk PC patients treated at the GSI (Gesellschaft für Schwerionenforschung) and the University of Heidelberg in an ongoing clinical phase I/II trial using combined photon intensity modulated radiation therapy (IMRT) and 12C carbon ion boost. Material and methods. Fourteen patients (planned accrual: 31 pts) have been treated within this trial so far. IMRT is prescribed to the median PTV at a dose of 30 × 2 Gy; 12C boost is applied to the prostate (GTV) at a dose of 6 × 3 GyE using raster scan technique. Safety margins added to the clinical target volume were determined individually for each patient based on five independent planning computed tomography (CT)-scans. Acute gastrointestinal (GI) and genitourinary (GU) toxicity was assessed and documented according to the CTCAE Version 3.0. Results. Radiotherapy was very well tolerated without any grade 3 or higher toxicity. Acute anal bleeding grade 2 was observed in 2/14 patients. Rectal tenesmus grade 1 was reported by three other patients. No further GI symptoms have been observed. Most common acute symptoms during radiotherapy were nocturia and dysuria CTC grade 1 and 2 (12/14). There was no severe acute GU toxicity. Conclusion. The combination of photon IMRT and carbon ion boost is feasible in patients with intermediate-risk PC. So far, the treatment has been well tolerated. Acute toxicity rates were in good accordance with data reported for high dose IMRT alone.
Recent studies showed that dose escalation to doses exceeding 76 Gy leads to improved local control and biochemical relapse free survival in patients with intermediate- and high-risk locally advanced prostate cancer [Citation1]. Delivery of high tumor doses is limited by the close proximity of the prostate to the anterior rectal wall and the urinary bladder. Using conventional conformal photon radiation therapy (RT), the anterior rectal wall is included in the target volume and the risk for severe toxicity to the rectum is known to increase with dose. Gastrointestinal (GI) late reactions ≥grade 2 were reported in about 15% (actuarial rate at five years after RT) of the patients treated with doses of 75.6–81 Gy [Citation2]. Genitourinary (GU) late effects ≥ grade 2 occurred in 10% of the patients at six years after 70–78 Gy.
Modern RT techniques such as intensity modulated RT (IMRT) allow sparing of the rectum during treatment. The clinical benefit of photon IMRT in prostate cancer patients has most recently been investigated within clinical studies [Citation3,Citation4].
Heavy charged particles provide the physical advantage of an inverted dose profile creating steep dose gradients. Adjacent organs at risk can be spared better than with photon radiation alone. Most experience with particle therapy for prostate cancer has been explored with protons. Initial results are promising [Citation5,Citation6]. At the National Institute of Radiological Sciences (NIRS) in Japan, patients with localized prostate cancer have been treated with carbon ions alone using passive beam modulation since 1995. Especially patients of the intermediate- and high-risk groups seemed to benefit from carbon ion radiotherapy [Citation7] with favorable toxicity [Citation8].
Between 1997 and 2007, carbon ion radiotherapy was available for patient treatments at the Gesellschaft für Schwerionenforschung (GSI). In contrast to the Japanese centers, the facility at GSI relied on active beam delivery using the raster scan technique, offering not only physical advantages with improved sparing of normal tissue in the beam entrance channel but also enabling biological plan optimization [Citation9,Citation10].
This publication reports initial results on acute side effects of an ongoing clinical phase I/II trial of combined photon IMRT and carbon ion boost in active beam scanning technique. The interim report of the first 14 patients with intermediate-risk prostate cancer treated between 2006 and 2008 at the GSI and the University of Heidelberg is presented.
Material and methods
Study design and patient characteristics
The trial was approved by the Ethics committee of the University of Heidelberg and the Bundesamt für Strahlenschutz (the governmental authority for radiation protection in Germany). The study is designed as a single arm prospective clinical phase I/II study investigating feasibility and toxicity of combined photon IMRT and carbon ion boost in patients with intermediate-risk prostate cancer. Feasibility and toxicity are evaluated using a Simon's two-stage design [Citation11]. A first analysis of feasibility and toxicity was planned to be carried out after inclusion of nine of the projected number of 31 patients. Acute radiation induced toxicity in more than seven patients of nine patients was defined as a stopping criterion for the trial. The study has to be terminated after inclusion of 14 patients, if ≥2 patients develop >grade 2 toxicities.
Eligibility
Inclusion criteria were age 50–75 years, a KPS (Karnofsky Performance Score) of at least 70% and a intermediate-risk prostate cancer (adenocarcinoma proven by sextant biopsy) with a serum PSA between 10.1 ng/ml and 20 ng/ml or at least T2b tumor or a Gleason Score of at least 7. Patient details are summarized in . Patients with lymph node or distant metastases were excluded as were patients with a history of former RT to the pelvis, chemotherapy, prior prostatectomy, hip endoprosthesis, inflammatory bowel disease or incontinence. Furthermore, patients with a history of other malignant tumors with a disease-free interval of less than five years, incomplete staging and inability to understand the aims of the study were excluded. The large prostate volume or dysfunction of urinary flow was not considered as exclusion criteria. The primary endpoints of the study were the feasibility and toxicity of combined photon IMRT and a carbon ion boost.
Table I. Patient characteristics.
Radiation therapy
The study was concepted to apply the photon IMRT with a total target dose of 60 Gy prescribed to the median dose of the planning target volume (PTV, weekly fractionation 5 × 2.0 Gy) and a carbon ion boost with a total boost dose of 18 GyE (weekly fractionation 6 × 3 GyE) to the prostate (GTV).
The first 14 patients included in the study were analyzed with respect to acute toxicity. The median age of the patients at initiation of the treatment was 68 years (range 55–75). Neoadjuvant hormonal therapy with LHRH-analoga was encouraged according to current recommendations in intermediate-risk patients with unfavorable factors, but was not part of the study protocol.
Treatment planning
Patients were immobilized in a rigid immobilization device consisting of the custom-made wrap-around body cast and a separate head mask to avoid set-up errors of >5 mm [Citation12]. Five independent planning computed tomography (CT)-scans were carried out on five consecutive days prior to treatment start. The safety margins added to the gross target volume (GTV) and clinical target volume (CTV) were determined individually for each patient based on these five planning scans. The CT-scans consisted of continuous 3 mm slices, which were obtained in a stereotactic set-up.
Organs at risk such as rectum, bladder and femoral heads were contoured individually in all five CT-scans.
The GTV, as defined for carbon ion RT, included the whole prostate with a safety margin of 3 mm anteriorly and laterally and were extended to contain at least the base of the seminal vesicles in tumors with infiltration of the seminal vesicles. The safety margin towards the rectum was defined according to the individual set-up variance of every patient determined from repeated control CT-scans.
The CTV for photon IMRT contains the GTV plus 5 mm in lateral direction (there was no margin towards OAR since T4 stages were excluded from the study) and the seminal vesicles. The PTVs for boost and for photons were derived from the individual variance of the GTV and the CTV derived from the repeated CT-scans. An overlap of the CTV and the rectal wall and the bladder was avoided to allow treatment plan optimization with inverse treatment planning for IMRT and to facilitate the dose constraints to the rectum for IMRT treatment planning. However, there was a small overlap of the PTV and the rectal wall and bladder. Dose constraints for the rectal wall were ≤ 68 GyE to anterior wall with 1% of the rectal wall volume allowed to receive doses > 68 GyE for combined treatment plan. Rectum was defined as rectum including the wall and its contents from the recto-sigmoid flexure (cranial edge) to the anal canal (caudal edge). The other dose constraints were defined as follows: posterior rectal wall ≤ 60 GyE, up to 50% of the bladder volume > 68 GyE, femoral heads <60 GyE.
Set-up errors were quantitatively assessed with CT-scans at least once a week during IMRT and by daily comparison of orthogonal x-rays to DRRs during carbon ion RT. Mean set-up deviation using a rigid body cast with an additional head mask system as determined in patients treated with photon IMRT for prostate cancer to be less than 3 mm [Citation12].
Carbon ion RT planning was performed using the treatment planning software TRiP including biological plan optimization [Citation10]. Biologically effective dose distributions were calculated using the a/ß ratio for prostate cancer (α/β = 2) as well as for the endpoint late toxicity to the rectum. Two opposing lateral fields were chosen. The intensity-controlled raster scan system was used for active beam application.
Dose specification was based on biological effective dose due to the high relative biologic effectiveness (RBE) of carbon ions, which differs throughout the target volume due to its dependence on various factors [Citation10,Citation13]. RBE was calculated at each voxel throughout the target volumes and biological optimization was performed. The dose prescription used was related to the biological dose GyE using daily fractions of 3 GyE.
Photon IMRT treatment plans were calculated using the treatment planning software KonRad [Citation14]. Five to seven coplanar fields were used for IMRT application in step-and-shoot technique at a 6 MV linear accelerator. A target dose of 60 Gy to the PTV for photons was prescribed to the median dose of the calculated dose distributions. Treatment planning aimed in the coverage of the PTVs by the 90%-isodose line anteriorly and laterally. The bladder volume treated to high doses was minimized, but no compromises were made with respect to the target coverage.
Dosimetric plan verification for both carbon ion and photon IMRT plans were performed for each plan prior to the treatment application.
Treatment delivery and assessment of acute toxicity
From February 2006 until July 2008, 14 patients of 31 projected patients have been treated within this study. Carbon ion therapy was performed at GSI Darmstadt within therapy blocks of 20 days three times a year. Photon IMRT was delivered at the Department of Radiation Oncology of the University of Heidelberg. Unfortunately, recruitment of the patients was slow due to limited availability of the carbon ion therapy slots at the GSI. In July 2008, the treatment cave at the GSI was closed for patient treatments. The treatment of the remaining study population will be done at a hospital-based particle therapy facility in Heidelberg that started clinical operation in 2009.
Acute gastrointestinal (GI) and genitourinary (GU) toxicity was assessed and documented according to the CTCAE Version 3.0 at predefined time points: weekly during RT, at the end of the RT series, six weeks after completion of RT and six months after RT. Further follow-up examinations were scheduled every six months, thereafter, for at least three years. Assessment of quality of life using EORTC questionnaires (EORTC QLQ-PR 25 and EORTC QLQ-C30) was also performed and results will be analyzed after completion of the trial. The biochemical recurrences were evaluated using the Phoenix criteria [Citation15].
Results
Median follow-up after treatment is 28 months (12–36 months). Radiation therapy was tolerated well. All 14 patients completed the treatment as planned. There was an interruption of the treatment for one week in patient number 2 because of acute sinusitis. There was no further therapy delay.
Only two of 14 patients (patient number 4 and 9) did not receive antihormonal therapy, though antihormonal therapy was not a subject of this study. Most of the patients received antihormonal therapy for at least 12 months.
Concerning other toxicities after RT two patients received a hip prosthesis which was already planned before RT because of pre-existing degenerative disease. Another patient was diagnosed with a glioblastoma and was treated with combination of radiation therapy and chemotherapy. This patient was further followed up within the prostate trial.
Acute toxicity
Radiation therapy was very well tolerated. No grade ≥3 toxicity occurred. Two of the patients developed acute anal bleeding with moderate pain due to hemorrhoidal problems CTC grade 1. Rectal tenesmus CTC grad 1 was reported by three other patients. No further gastrointestinal symptoms were observed. One patient underwent screening colonoscopy one year after irradiation showing only small angiodysplasies within the prior RT volume.
Most common acute genitourinary symptoms during radiation therapy were nocturia and dysuria. Seven patients (50%) developed acute genitourinary toxicity CTC grade 1, and five of 14 patients (36%) showed acute grade 2 toxicity. Restitution of acute symptoms was observed in the majority (12/14) of the patients at first follow-up. No patient developed severe acute genitourinary toxicity. We did not observe any unexpected acute side effects of combined photon and carbon ion RT (see ).
Table II. Detailed data of acute toxicity.
PSA-response and survival
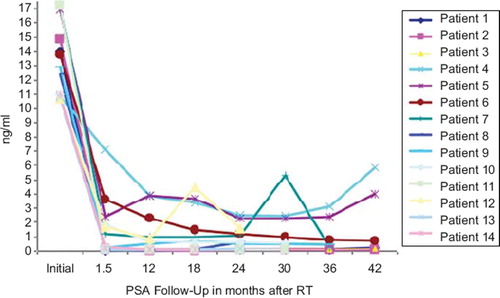
show the PSA data for all patients included in the trial so far. Two biochemical recurrences have been recorded (patient 4 and 7): patient 4 had a cholin PET examination showing no evidence of lymph node or distant metastasis; salvage therapy consisted of complete androgen blockade. In patient 7, only an FDG-PET scan was available without hint of distant metastases, he received bicalutamid as salvage therapy.
Intermittent PSA level elevation was found in patient number 12: the initial mean PSA level was 13.2 ng/ml (range 10.7–17.2 ng/ml). The mean PSA nadir 1.5 years after irradiation was 1.14 ng/ml (range 0.1–2.5 ng/ml).
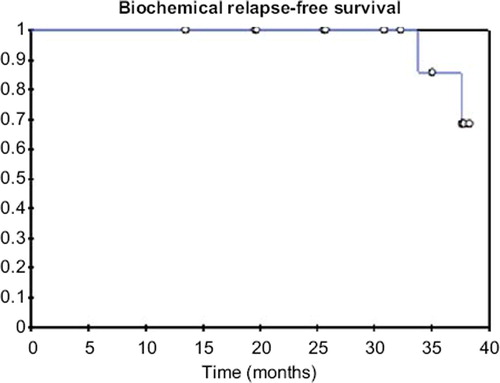
The actuarial three-year overall survival in the whole cohort was 100%. The actuarial three-year biochemical relapse free survival was 86% (). The one-year distant metastases free survival was 100%.
Discussion
The treatment of intermediate-risk prostate cancer is still challenging. Promising results are reported using proton therapy. In intermediate-risk prostate cancer with a PSA between 10.1 ng/ml and 20 ng/ml, biochemical control rates of 67% at four years were achieved with protons compared to 58% with photons and 56% after prostatectomy, respectively [Citation5]. Probably due to short follow-up, influences of overall survival could not be demonstrated [Citation6].
The experience in carbon ion therapy for prostate cancer is mainly based on the work of our Japanese colleagues. Prescribed doses for prostate cancer treatment at NIRS were 54–72 GyE in a clinical phase I/II trial and were fixed to 66 GyE/20 fractions in the ongoing clinical phase II trial. The biochemical recurrence free survival was 82.4% at five years in 96 patients with T1b-3 tumors. Especially patients of the intermediate- and high-risk groups seemed to benefit from carbon ion RT. The biochemical disease free survival was 81.1% in patients with T2b-3 tumors and risk factors such as Gleason Score of 7 or higher and a pre-treatment PSA of 20 ng/ml or higher [Citation7]. Acute and late grade 2 GI reactions were observed in four patients (2%), GU grade 2 reactions only nine patients (5%). No Grade 3 or higher toxicity was registered [Citation8].
The present study reports the first treatment results using raster scanned carbon ions for intermediate-risk prostate cancer patients. In our trial of combined photon IMRT and carbon ion RT, a biologically equivalent dose of 82.5 GyE was delivered in all patients assuming an α/β of 2 for prostate cancer and daily standard fraction dose of 2 Gy. We did not observe any severe acute toxicity. Acute grade 2 GU toxicity was observed in five (36%) of 14 patients and grade 2 GI toxicity not found at all.
As reported by Matzinger et al., photon RT in prostate cancer up to 78 Gy was well tolerated [Citation16]. The authors report Dmax-bladder and D50%-rectum as factors influencing the risk for grade 2 GU and GI toxicity, respectively. Both values were lower with IMRT in our patient group. Acute GU toxicity CTC grade 2 was observed in 22% of the patients receiving ultra-high dose IMRT with 86.4 Gy. Acute GI toxicity CTC grade 2 was observed in 8% of the patients after IMRT [Citation4]. Furthermore, Cahlon et al. report grade 3 GU and GI toxicity in 0.6% of their patients [Citation4]. Acute toxicity rates after dose escalation of proton therapy up to 82 GyE were published by Coen et al. The reported GI/ GU acute toxicity rate were 50% for grade 1, 14% for grade 2 and 1% for grade 3 toxicity, respectively [Citation18]. The toxicity rates in our trial indicate that acute toxicity after combined photon IMRT and carbon ion RT is not higher than expected at this dose level using photon IMRT or proton radiation alone.
Based on the assumption that prostate cancer cells show a low α/β ratio [Citation19], hypofractionation with carbon ion therapy has became a topic of high interest. Thames et al. proposed that a decrease of the overall treatment time for the prostate cancer patients receiving more than 70 Gy can improve outcome [Citation20]. First results of hypofractionated carbon ion RT alone in prostate cancer patients are already available. After a total dose of 66 GyE delivered in 16 fractions within four weeks high five-year bDFS (biochemical disease free survival) rates have been observed with low toxicity rates [Citation7,Citation8]. Currently, further reduction of overall treatment time for carbon ion RT in prostate cancer to 10 days is investigated at the NIRS in Chiba.
The major advantage of carbon ion treatment delivery in our trial in comparison to Japanese studies is the active beam application using raster scanning. In our trial, we could show that combined photon IMRT and a carbon ion boost with six fractions of 3 GyE is feasible and well tolerated. A low rate of toxicity in the present study is the premise for further dose escalation and hypofractionation trials.
Within several planning inter-comparison studies we determined the optimal treatment with respect to safety and quality of dose distributions to be a combination of photon IMRT and a carbon ion boost. Optimal beam directions for the carbon ion boost were two opposing lateral fields. Using this technique, the physical and biologic advantages of carbon ions are assumed to be fully exploited. At the same time the influence of set-up errors within a rigid immobilization device is minimal. With respect to the higher sensitivity of scanned carbon ions to small set-up errors, a combined therapy approach is applied. Furthermore, an excellent coverage of the target volume could be achieved with carbon ion RT while optimally sparing the rectum [Citation21].
We used several CT-scans to define our PTV in order to account for different organ fillings. Thus, we investigated the individual characteristics of internal organ motion for each patient before the irradiation and created the patient-specific target volumes. A small overlap region of the planning target volume for carbon ion boost and the rectum was allowed. We made no compromise of the coverage due to sparing of the bladder. This target definition concept was feasible and clinically reasonable. However, the further investigations in contouring process has to be considered giving better possibility of outlining the organs at risk as described by Thörnqvist et al. [Citation22].
The present study has been initiated before adaptive radiation therapy was widely accepted and cone beam CT imaging became an integral part of radiation therapy [Citation23]. During carbon ion RT at GSI set-up was checked daily with a system based on orthogonal x-rays and set-up deviations >3 mm are corrected prior to RT. As mentioned above, the study will be continued at Heidelberg Ion-Beam Therapy Center (HIT) in the same manner with daily positioning control. As soon as the option of cone beam CT will be available, we will be able to switch to the adaptive approach in carbon ion therapy.
In the present trial, an intermittent PSA level elevation was found in patient number 12. We assumed that it was most likely due to sexual activity just before PSA determination. seven-year PSA-relapse free survival for intermediate-risk patients of 72% has been reported [Citation17]. The three-year biochemical disease free survival rate in our trial was 86%, which is comparable to data published for IMRT alone. However, the follow-up is too short and the patient number is too small to draw conclusions with respect to outcome.
Neoadjuvant antihormonal therapy has been added in 12 of 14 patients in our trial. Since antihormonal therapy has been shown to have an impact on outcome in intermediate-risk patients [Citation24], the outcome results will also have to be discussed with respect to this issue in the future.
In conclusion, the combination of photon IMRT and carbon ion boost is feasible in patients with intermediate-risk prostate cancer. The treatment was well tolerated. There was no toxicity > CTC grade 2. Acute toxicity rates were in good accordance with acute toxicity data reported for high dose IMRT alone. As a consequence, we will proceed with the recruitment of patients into the trial as planned. Initial PSA control data indicates a good effectiveness of the treatment as well, but follow-up time is too short to draw any conclusions with respect to biochemical disease free survival or overall survival.
Acknowledgements
The development of the study protocol has been supported and reviewed by the European Network for light ion hadrontherapy (ENLIGHT) with EU support. The study has been supported by the Sander Stiftung, Germany. There is no conflict of interest to be declared.
References
- Kuban DA, Levy LB, Cheung MR, Lee AK, Choi S, Frank S, . Long-term failure patterns and survival in a randomized dose-escalation trial for prostate cancer. Who dies of disease? Int J Radiat Oncol Biol Phys 2011;79:1310–7.
- Zelefsky MJ, Cowen D, Fuks Z, Shike M, Burman C, Jackson A, . Long term tolerance of high dose three-dimensional conformal radiotherapy in patients with localized prostate carcinoma. Cancer 1999;85:2460–8.
- Zelefsky MJ, Fuks Z, Hunt M, Yamada Y, Marion C, Ling CC, . High-dose intensity modulated radiation therapy for prostate cancer: Early toxicity and biochemical outcome in 772 patients. Int J Radiat Oncol Biol Phys 2002;53:1111–6.
- Cahlon O, Zelefsky MJ, Shippy A, Chan H, Fuks Z, Yamada Y, . Ultra-high dose (86.4 Gy) IMRT for localized prostate cancer: Toxicity and biochemical outcomes. Int J Radiat Oncol Biol Phys 2008;71:330–7.
- Rossi CJ. Conformal proton beam therapy of prostate cancer – update on the Loma Linda University medical center experience. Strahlenther Onkol 1999;175(Suppl 2): 82–4.
- Zietman AL, Bae K, Slater JD, Shipley WU, Efstathiou JA, Coen JJ, . Randomized trial comparing conventional-dose with high-dose conformal radiation therapy in early-stage adenocarcinoma of the prostate: Long-term results from proton radiation oncology group/American College of Radiology 95-09. J Clin Oncol 2010;28:1106–11.
- Akakura K, Tsujii H, Morita S, Tsuji H, Yagishita T, Isaka S, . Phase I/II clinical trials of carbon ion therapy for prostate cancer. Prostate 2004;58:252–8.
- Ishikawa H, Tsuji H, Kamada T, Yanagi T, Mizoe JE, Kanai T, . Carbon ion radiation therapy for prostate cancer: Results of a prospective phase II study. Radiother Oncol 2006;81:57–64.
- Haberer T, Becher W, Schardt D, Kraft G. Magnetic scanning system for heavy ion therapy. Nucl Instr Meth Phys Res 1993;330:296–305.
- Krämer M, Jäkel O, Haberer T, Rietzel E, Schardt D, Scholz M, . Treatment planning for scanned ion beams. Radiother Oncol 2004;73(Suppl 2):S80–5.
- Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trial 1989;10:1–10.
- Herfarth KK, Pirzkall A, Lohr F, Schulz-Ertner D, Spoo J, Frank C, . First experiences with a noninvasive patient set-up system for radiotherapy of the prostate. Strahlenther Onkol 2000;176:217–22.
- Kraft G. RBE and its interpretation. Strahlenther Onkol 1999;175(Suppl II):44–7.
- Preiser K, Bortfeld T, Hartwig K, Schlegel W, Stein J. Inverse radiotherapy planning for intensity modulated photon fields. Radiologe 1998;38:228–34.
- Abramowitz MC, Li T, Buyyounouski MK, Ross E, Uzzo RG, Pollack A, . The Phoenix definition of biochemical failure predicts for overall survival in patients with prostate cancer. Cancer 2008;112:55–60.
- Matzinger O, Duclos F, van den Bergh A, Carrie C, Villà S, Kitsios P, . EORTC Radiation Oncology Group. Acute toxicity of curative radiotherapy for intermediate- and high-risk localised prostate cancer in the EORTC trial 22991. Eur J Cancer 2009;45:2825–34.
- Zelefsky MJ, Levin EJ, Hunt M, Yamada Y, Shippy AM, Jackson A, . Incidence of late rectal and urinary toxicities after three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys 2008;70:1124–9.
- Coen JJ, Bae K, Zietman AL, Patel B, Shipley WU, Slater JD, . Acute and late toxicity after dose escalation to 82 GyEGyEGyE using conformal proton radiation for localized prostate cancer: Initial Report of American College of Radiology Phase II Study 03-12. Int J Radiat Oncol Biol Phys Epub 2010 Oct 5.
- Fowler J, Chappell R, Ritter M. Is a/ß for prostate tumors really low? Int J Radiat Oncol Biol Phys 2001;50:1021–31.
- Thames HD, Kuban D, Levy LB, Horwitz EM, Kupelian P, Martinez A, . The role of overall treatment time in the outcome of radiotherapy of prostate cancer: An analysis of biochemical failure in 4839 men treated between 1987 and 1995. Radiother Oncol 2010;96:6–12.
- Nikoghosyan A, Schulz-Ertner D, Didinger B, Jäkel O, Zuna I, Höss A, . Evaluation of the therapeutical potential of heavy ion therapy for patients with locally advanced prostate cancer. Int J Radiat Oncol Biol Phys 2004;58:89–97.
- Thörnqvist S, Petersen JB, Høyer M, Bentzen LN, Muren LP. Propagation of target and organ at risk contours in radiotherapy of prostate cancer using deformable image registration. Acta Oncol 2010;49:1023–32.
- Søvik A, Rødal J, Skogmo HK, Lervåg C, Eilertsen K, Malinen E. Adaptive radiotherapy based on contrast enhanced cone beam CT imaging. Acta Oncol 2010;49: 972–7.
- Bolla M, de Reijke TM, Van Tienhoven G, Van den Bergh AC, Oddens J, Poortmans PM, . EORTC Radiation Oncology Group and Genito-Urinary Tract Cancer Group. Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med 2009;360:2516–27.