Abstract
Background. Several studies have reported an association between breast cancer unit volume and prognosis. We hypothesize that this may be due to inappropriate coping with the recommended guidelines for adjuvant therapy rather than improper breast cancer surgery provided at smaller units. Methods. A cohort of 1131 patients with operable breast cancer (pT1-2 and positive axillary lymph nodes, stage II) enrolled between 1984 and 1994 were analyzed. The women had participated in one of three prospective trials on adjuvant endocrine treatment and were enrolled from 50 centers in Norway. The hospitals were categorized into four groups according to the annual number of surgically treated breast cancer patients reported to the national discharge database in 1990. The hospitals were also stratified according to whether they are university or non-university hospitals. To assess the effect of unit size on patient outcome, local recurrence rates and overall survival were compared in women treated at units with different patient volumes. Results. The median time from study enrolment to the end of the study was 10.5 years. Relapse-free survival and overall survival did not differ significantly between the hospital groups based on the surgical workload or between university and non-university hospitals. Conclusions. Patient volume or teaching status of a hospital did not have any impact on the prognosis of pre- or postmenopausal stage II breast cancer patients included in the adjuvant endocrine trials. Our data support the hypothesis that differences in survival related to patient volume at the treatment units may be explained by inappropriate adjuvant systemic treatment.
The implementation of adjuvant therapy and, most likely, mammographic screening has improved breast cancer survival in Western countries [Citation1–4]. Following the seminal report by Sainsbury et al. in 1995 [Citation5] reporting an inferior outcome among patients treated in small surgical units, several reports have confirmed their findings [Citation6–11]. Outcomes have been related to the individual surgeons, hospital patient volume [Citation8], and whether treatment was administered at a cancer center or in a general hospital. In contrast, a previous study from Norway comparing university and non-university hospitals [Citation12], and one study from the US comparing larger and smaller surgical units [Citation13], both revealed no difference in the outcomes in relation to case volume. However, both studies enrolled unselected patients, and any systematic variation with respect to medical therapy may not be accounted for.
We hypothesize that any differences regarding outcome may reflect suboptimal adjuvant therapy in smaller units due to a lack of adherence to recommended guidelines. Thus, we aimed to address two questions: 1) whether there are any differences in the relapse-free survival (RFS) and overall survival (OS) of patients treated at smaller versus larger hospitals, and 2) whether there are any differences in outcome between breast cancer patients surgically treated at university versus non-university hospitals. To address these questions, we evaluated the long-term outcomes of 1131 women with operable breast cancer enrolled in three different adjuvant endocrine treatment trials and for whom individual adherence to adjuvant therapy was confirmed.
Material and methods
Patients
Individual primary patient data from three prospective multicenter randomized trials [Citation14–16] conducted by the Norwegian Breast Cancer Group (NBCG) between 1984 and 1994 were used (). All patients had operable (pT1-2) axillary node-positive (pN+), oestrogen (ER) and/ or progesterone receptor (PgR)-positive, or receptor unknown breast cancer. Three hundred and seventy, 320, and 489 patients were enrolled between January 1984 and February 1988 (study 1), January 1989 and June 1994 (study 2), and March 1989 and December 1994 (study 3), providing a total of 1179 patients of whom 1131 (96%) were eligible. The 48 ineligible were included by mistake, i.e. protocol violation. The main reasons for being ineligible were other malignancies, node negativity or too large tumors (T3). At inclusion, the patients were less than 75 years of age (NBCG study 1), less than 50 years of age (study 2), and between 50 and 75 years of age (study 3) (). Patient and tumor characteristics are shown in .
Table I. Number of eligible patients included in three multicenter randomized adjuvant trials of breast cancer in Norway 1984–1994.
Table II. Patient and tumour characteristics of evaluable patients randomized into adjuvant trials of breast cancer in Norway 1984–1994.
The Regional Committee of Ethics in Oslo approved the three studies comprising this investigation, and informed consent was obtained from all included patients.
Treatment regimens and follow-up
Primary surgery included level I and II axillary lymph node dissection and either mastectomy (89%) or breast conservative surgery (11%). Chemo- and radiotherapy was employed according to national recommendations (NBCG, www.nbcg.no) at that time.
Adjuvant endocrine treatment () was tamoxifen versus control (study 1), tamoxifen versus luteinizing hormone-releasing hormone (LHRH) analogue (study 2), and tamoxifen versus sequential tamoxifen and megestrol acetate (MA) (study 3).
Follow-up included clinical examination every third month for two years, then every sixth month up to three years, and then annually thereafter until death, according to protocol [Citation14–16]. The median time from study enrolment to the end of the study was 10.5 years (range 6.6–14.1 years).
Tamoxifen was given as Nolvadex® 20 mg daily, LHRH analogue as Zoladex® 3.6 mg monthly, and MA as Megace® 160 mg daily. For the cyclic treatment arm (study 3), tamoxifen and MA were administered in alternating cycles of eight weeks. Adjuvant endocrine treatment was recommended and scheduled for two years. All patients had a normal chest x-ray and normal liver blood tests before inclusion in the study.
Tumour classification was done according to the UICC: TNM classification of malignant tumours, 3rd edition, revised 1982. The last follow-up was 31 December 1996, 15 February 2001, and 15 July 2002 for studies 1, 2, and 3, respectively.
A clinical follow-up form was completed at every visit. The initial sites of loco-regional recurrence or distant metastases were recorded when confirmed. Confirmation was made as follows: chest wall or regional nodes: cytology or histology whenever possible; bone: conventional radiology or bone scan; lungs: chest x-ray or computer tomography (CT) when appropriate; liver: ultrasound and/or CT, confirmed by cytology (FNAC) when necessary. Possible new ipsilateral cancers were included in the number of local recurrences.
Hospital volume and function
The inclusion of patients in the three studies varied considerably within single institutions, the number of patients included was not appropriate for the allocation of hospitals into size categories. Therefore, the SINTEF Unimed national discharge database for the year 1990 was used for categorizing the hospitals into groups according to the number of surgically treated breast cancer patients () [Citation17]. The year 1990 was considered representative for the time period (1984–1994) of these studies and, therefore, used as an index year.
In 1990, 64 hospitals offered surgical breast cancer treatment in our country [Citation17]. Thirty two, 35, and 36 hospitals enrolled patients in study 1, 2, and 3, respectively, with a total of 50 different hospitals with eligible patients included in the present study ().
For further evaluation of a possible impact of surgical volume on prognosis, the hospitals were categorized into various groups, such as 1–10, 11–30, 31–50, and 51+ number of yearly operated patients. Groups were also combined when analyzed. Each surgical department was also classified as an academic unit (university hospital) or a non-academic unit. Additional calculations and evaluations were done for hospitals with high inclusion rates (approximately 25% of the study population) versus those with a low inclusion rate (approximately 25% of the study population) (). Calculations for different age groups were also performed.
Table III. Survival data per operating volume (patients, pts) and hospital function category for patients randomised into adjuvant trials of breast cancer in Norway 1984–1994, with number of patients (n) in each category.
Statistical methods
Descriptive statistics were presented as medians (range). The RFS and OS were estimated using the Kaplan-Meier method, and the groups were compared by log-rank tests. The expected important factors were included in the Cox proportional hazards regression model, and the analyses were performed with backward stepwise elimination. P-values < 0.05 were considered significant. SPSS 15.0 for Windows was used for statistical calculations.
Results
A positive hormone receptor status (ER + and/or PgR+) for the primary breast cancer was confirmed in 959 patients (85%) and an unknown receptor status in the remaining 172 patients.
The median number of lymph nodes removed was 10 for high volume (n ≥ 50) units and university hospitals versus 8 for low volume (n = 1–50) units and non-university hospitals (p < 0.001). The median number of involved nodes was 2 (range 1–32), irrespective of annual treatment volume and type of hospital. However, the proportion of patients with ≥ 4 axillary lymph node metastases was significantly higher at university hospitals (34%) compared to low volume hospitals (26%, p = 0.01). Other variables, including tumor diameter, were evenly distributed between the defined groups ().
At last follow-up (median 9 years, range 2–14 years), recurrent disease was diagnosed in 567 patients, and 482 patients had died. The median follow-up for 491 patients still alive without relapse of disease was 8.4 years (range 1.9–13.8 years), and 9.5 years (range 5.3–13.7 years) for 147 patients still alive at last follow-up but with confirmed recurrent disease. In total, 145 patents had their first recurrence on the chest wall or in the ipsilateral breast.
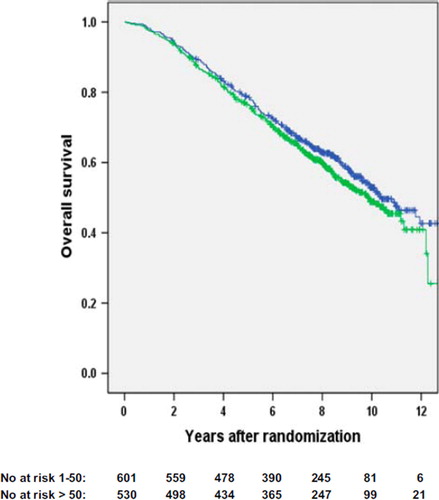
Univariate analyses with regard to RFS, cancer-specific survival (data not shown), and OS did not show any significant outcome differences according to surgical volume groupings, which was also true with regard to university and non-university hospitals (). Neither did univariate analyses on subgroups (study 1, 2, and 3; period of entrance, type of endocrine treatment if given, age, type of surgery, T-category, N-category) show survival differences ( and ).
Figure 1. Recurrence-free survival for high volume surgical units (> 50 patients/year, black line) versus low volume surgical units (1–50 patients/year, grey line). The number of patients still at risk is provided below the figure (p = 0.8).
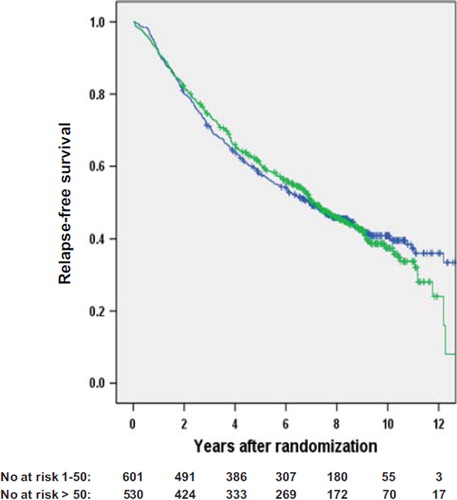
Figure 2. Overall survival for high volume surgical units (> 50 patients/year, black line) versus low volume surgical units (1–50 patients/year, grey line). The number of patients still at risk is provided below the figure (p = 0.2).
For OS, the following variables were included in the Cox proportional hazard regression model: Operating volume (1–30 vs. > 30 per year, or 1–50 vs. > 50 per year), age group, type of surgery (mastectomy vs. lumpectomy), study number (1, 2, and 3), T category (T1 vs. T2), lymph node metastases group (1–3 vs. > 3 positive lymph nodes), and endocrine treatment (yes vs. no; no only applying to the control group in study 1). The variables study number (the older patients in study 3 having the worst outcome), T category, lymph node metastasis group, and endocrine treatment remained in the final model. Operating volume did not remain in the final model.
The same variables were included in the Cox proportional hazard regression model for RFS. In the final model, these variables remained: T category, lymph node metastasis group, and endocrine treatment. Operating volume did not remain in the final model.
Because the control group (n = 170) in study 1 did not receive adjuvant endocrine therapy, a separate univariate analysis of RFS and OS was performed without these 170 patients. No differences between high and low volume hospitals were found (data not shown).
43% of the patients received chemotherapy, and there was no difference between high and low volume hospitals.
Radiotherapy was given to 30% of the randomized patients, and significantly more patients in high volume hospitals received radiotherapy.
There were no survival differences in the various age groups as + 50 and + 60 years, stratified on hospital size.
Discussion
The reported inferior outcome in low volume hospitals [Citation5–10] has been explained, in part, by inadequate surgery. In addition, concerns have been raised regarding inappropriate or no adjuvant treatment at smaller units. Endocrine treatment and chemotherapy may each reduce the relapse rate and potential death from breast cancer by at least 30% if the patients are properly selected. Combining adjuvant chemotherapy and endocrine treatment may reduce breast cancer mortality by up to 50% [Citation18]. Thus, quality control programs become imperative.
To our knowledge, this is the first paper examining outcomes among breast cancer patients treated according to prospective protocols with respect to type of center and surgical work load.
At the time of inclusion in these protocols, breast cancer surgery was performed at 67 hospitals in Norway [Citation17]. Consequently, the annual number of breast cancer patients treated in many units was low. This situation is mirrored in many other European countries and is encountered in many areas worldwide [Citation11,Citation19].
The fact that many of these low volume units participated in ongoing controlled, randomized trials enabled an evaluation of the outcomes of patients treated at small units, i.e. conducted within the study protocol and a quality control program with respect to compliance to adjuvant therapy. Fifty-two hospitals enrolled patients into one or more of the adjuvant trials.
The high number of hospitals joining the trials reveals a high standard of the surgical services, because all patients were taken care of and randomized by the general surgeon in charge of the patient.
Concerning the treatment paths (treatment guidelines by NBCG) of breast cancer patients, guidelines advised that all node-positive patients were given anti-oestrogens (tamoxifen) and short-term chemotherapy. There were no national differences in treatment paths.
Less than one-third of patients received radiotherapy, and this was more widely applied in high volume hospitals. If this should have impact on prognosis, small hospitals would be expected to do worse, which they did not.
Regarding the representative nature of these randomized patients versus the entire breast cancer population during the same period of time, we found according to the Norwegian Cancer Registry (personal communication, Gry Skare) that a total of 3300 patients were eligible for the protocols based on their breast disease alone (stage and receptor status). Assuming that non-eligibility for various medical reasons or no consent may affect 10% (low estimate) of these patients, the recruitment of a total number of 1179 patients is approaching 40%, which is much higher than the 3–14% enrolled in similar studies in other countries [Citation20,Citation21]. The issue of selection of patients, based on these numbers, is assumed to be of minor importance.
Whether participation in a clinical trial may influence outcome has been addressed by other studies. In two large meta-analyses [Citation22,Citation23] including trials for the treatment of various malignancies, outcomes were similar in trial participants compared to non-participants. In these reports, the non-participants were recruited partly from the same hospitals and partly on a population basis, suggesting that they were treated at the same level of professional skill and (should be) anticipated to receive similar therapy whether enrolled in studies or not.
Our data support that strict guidelines for adjuvant therapy are more important than hospital volume per se to achieve high quality standards in breast cancer treatment. This interpretation is in line with suggestions made by others, and in line with EUSOMA guidelines [Citation22–24]. Thus, the results should not be interpreted in favor of an ignorant attitude towards surgical demands, but rather to highlight the importance of appropriate management and care for patients with respect to systemic therapy.
The high volume hospitals had more patients with > 3 positive nodes (). An explanation for the greater number of involved nodes in high volume hospitals may be the higher total number (10 vs. 8) of nodes removed. The higher number of removed nodes makes it more likely that a higher number of involved nodes may be found, increasing the proportion of node-positive patients with > 3 lymph nodes involved. This interpretation is supported by Raabe et al. [Citation25]. In contrast to that study, others have claimed that an increasing number of uninvolved nodes are associated with a decreased risk of recurrence [Citation26]. These potential effects may balance each other, and it is not likely that these differences may influence the outcome in any direction to a major extent.
We did not observe any benefit for women treated at high volume hospitals compared to low volume hospitals. The discrepancy between our observations and the findings reported by others [Citation9–13,Citation19,Citation27] can most likely be explained by an improved and consistent adherence to nationwide guidelines (NBCG) at the hospitals involved in our three national prospective adjuvant trials. In addition, we suggest that the surveillance and data logistics imposed by the trial committees were important. Therefore, we would argue for stringent guidelines and a national quality assurance system to ensure that the guidelines are adhered to by all hospitals responsible for breast cancer treatment.
Contributors
Conception and design: HE Fjösne, R Kåresen, S Lundgren
Provision of study patients: HE Fjösne, JA Søreide, R Kåresen, PE Lønning, S Lundgren
Collection/assembly of data: HE Fjösne, A-B Jacobsen, S Lundgren
Data analysis and interpretation: HE Fjösne, JA Søreide, R Kåresen, PE Lønning, A-B Jacobsen, S Lundgren
Manuscript writing: HE Fjösne, JA Søreide, R Kåresen, PE Lønning, A-B Jacobsen, S Lundgren
Final approval of manuscript: HE Fjösne, JA Søreide, R Kåresen, P E Lønning, A-B Jacobsen, S Lundgren
Acknowledgements
The study was financially supported by The Norwegian Cancer Society. We want to thank Einar Hannisdal, Eva Skovlund and the Clinical Research Unit at the Norwegian Radium Hospital, Oslo University Hospital for important help in randomization, data handling, and statistical analysis; Gry Skare at the Cancer Registry of Norway for estimating the amount of patients available for randomization in these trials; and all the physicians enrolling patients into this study.
The following hospitals (in alphabetical order in Norwegian) included patients in the three studies: Aker sh, Aust-Agder Ssh, Bærum sh, Buskerud Ssh, DNR, Diakonhjemmets sh, Diakonissehjemmets sh, Farsund sh, Fsh Florø, Flekkefjord sh, Fsh i Lærdal, Fsh i Molde, Fsh på Stord, Fsh på Voss, Fsh i Kristiansund, Fsh i Volda, Gjøvik Fsh, Hammerfest sh, Hamar sh, Halden sh, Harstad sh, Haugesund Fsh, Haukeland sh, Betanien sh, Indre Østfold sh, Innherred sh, Kirkenes sh, Kongsberg sh, Kongsvinger sh, Larvik sh, Lovisenberg sh, Lillehammer Fsh, Moss sh, Namdal sh, Nordland Ssh, Orkdal sh, Rana sh, RiTø, RiT, Ringerike sh, Sandnessjøen sh, Ssh i Møre og Romsdal, Ssh i Rogaland, Ssh i Sogn og Fjordane, Telemark Ssh, Ssh i Akershus, Stokmarknes sh, Tynset sh, Ullevål sh, Vest-Agder Ssh, Vestfold Ssh, Østfold Ssh.
No sponsors have influenced data interpretation or manuscript production. The corresponding author and two co-authors (ABJ and SL) had full access to all data. The corresponding author had final responsibility to submit the manuscript.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Cancer in Norway 2004 [Internet]. Cancer Registry of Norway. Oslo: Institute of Population-based Cancer Research; 2006. Available from http://www.cancerregistry.no
- Malvezzi M, Arfé A, Bertuccio P, Levi F, La Vecchia C, Negri E. European cancer mortality prediction for the year 2011. Ann Oncol 2011;22:947–56.
- Peto R, Boreham J, Clarke M, Davies C, Beral V. UK and USA breast cancer deaths down 25% in year 2000 at ages 20–69 years. Lancet 2000;355:1822.
- Cheung KL. Endocrine therapy of breast cancer: An overview. Breast 2007;16:327–43.
- Sainsbury R, Haward B, Rider L, Johnston C, Round C. Influence of clinician workload and patterns of treatment on survival from breast cancer. Lancet 1995;345:1265–70.
- Grilli R, Minozzi S, Tinazzi A, Labianca R, Sheldon TA, Liberati A. Do specialists do it better? The impact of specialization on the processes and outcomes of care for cancer patients. Ann Oncol 1998;9:365–74.
- Kingsmore D, Hole D, Gillis C. Why does specialist treatment of breast cancer improve survival? The role of surgical management. Br J Cancer 2004;90:1920–5.
- Richards M, Sainsbury R, Kerr D. Inequalities in breast cancer care and outcome. Br J Cancer 1997;76:634–8.
- Guller U, Safford S, Pietrobon R, Heberer M, Oertli D, Jain NB. High hospital volume is associated with better outcomes for breast cancer surgery: Analysis of 233,247 patients. World J Surg 2005;2:994–9; Discussion 999–1000.
- Skinner KA, Helsper JT, Deapen D, Ye W, Sposto R. Breast cancer: Do specialists make a difference? Ann Surg Oncol 2003;10:606–15.
- Stefoski Mikeljevic J, Haward RA, Johnston C, Sainsbury R, Forman D. Surgeon workload and survival from breast cancer. Br J Cancer 2003;89:487–91.
- Raabe NK, Kaaresen R, Fossaa SD. Hospital-related differences in breast cancer managment. Analysis of an unselected population-based series of 1353 radically operated patients. Breast Cancer Res Treat 1997;43:225–35.
- Harcourt KF, Hicks KL. Is there a relationship between case volume and survival in breast cancer? Am J Surg 2003;185: 407–10.
- Gundersen S, Hannisdal E, Søreide JA, Skarstein A, Varhaug JE. Adjuvant tamoxifen for pre- and postmenopausal women with estrogen receptor positive, node positive breast cancer: A randomized study. Breast Cancer Res Treat 1995;36:49–53.
- Söreide JA, Varhaug JA, Fjösne HE, Erikstein B, Jacobsen A-B, Skovlund E, . Adjuvant endocrine treatment (goserelin vs. tamoxifen) in pre-menopausal patients with operable node positive stage II breast cancer. Eur J Surg Oncol 2002;28: 505–10.
- Fjösne HE, Jacobsen A-B, Lundgren S. Adjuvant cyclic tamoxifen and megestrol acetate treatment in post-menopausal breast cancer patients – longterm follow-up. A prospective randomized national multicenter study. Eur J Surg Oncol 2007;34:6–12.
- Lundgren S, Jørgensen S, Kåresen R. Surgery for breast cancer patients in Norway 1990–95. Tidskr Nor Lægeforen 2001; 121:2688–93. Norwegian.
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet 2005;365:1687–717.
- Ingram DM, McEvoy SP, Byrne MJ, Fritschi L, Joseph DJ, Jamrozik K. Surgical caseload and outcomes for women with invasive breast cancer treated in Western Australia. Breast 2005;14:11–7.
- Johansen MA, Mayer DK, Hoover HC Jr. Obstacles to implementing cancer clinical trials. Semin Oncol Nurs 1991; 7:260–7.
- Lara PN Jr, Paterniti DA, Chiechi C, Turrell C, Morain C, Horan N, . Evaluation of factors affecting awareness of and willingness to participate in cancer clinical trials. J Clin Oncol 2005;23:9282–89.
- Vist GE, Hagen KB, Devereaux PJ, Bryant D, Kristoffersen DT, Oxman AD. Systematic review to determine whether participation in a trial influences outcome. Br Med J 2005;330:1175–9. Review.
- Peppercorn JM, Weeks JC, Cook EF, Joffe S. Comparison of outcomes in cancer patients treated within and outside clinical trials: Conceptual framework and structured review. Lancet 2004;363:263–70. Review.
- EUSOMA. The requirements of a specialist breast unit. Eur J Cancer 2000;36:2288–93.
- Raabe NK, Kaaresen R, Fosså SD. Analysis of adjuvant treatment in postmenopausal patients with stage II invasive breast carsinoma. A pattern of care study and quality assurance of 431 consecutive patients in Oslo. Acta Oncol 1997;36:255–60.
- Karlsson P, Cole BF, Price KN, Coates AS, Castiglione-Gertsch M, Gusterson BA, . The role of the number of uninvolved lymph nodes in predicting locoregional recurrence in breast cancer. J Clin Oncol 2007;25:2019–25.
- Basnett I, Gill M, Tobias JS. Variations in breast cancer management between a teaching and a non-teaching district. Eur J Cancer 1992;28A:1945–50.
